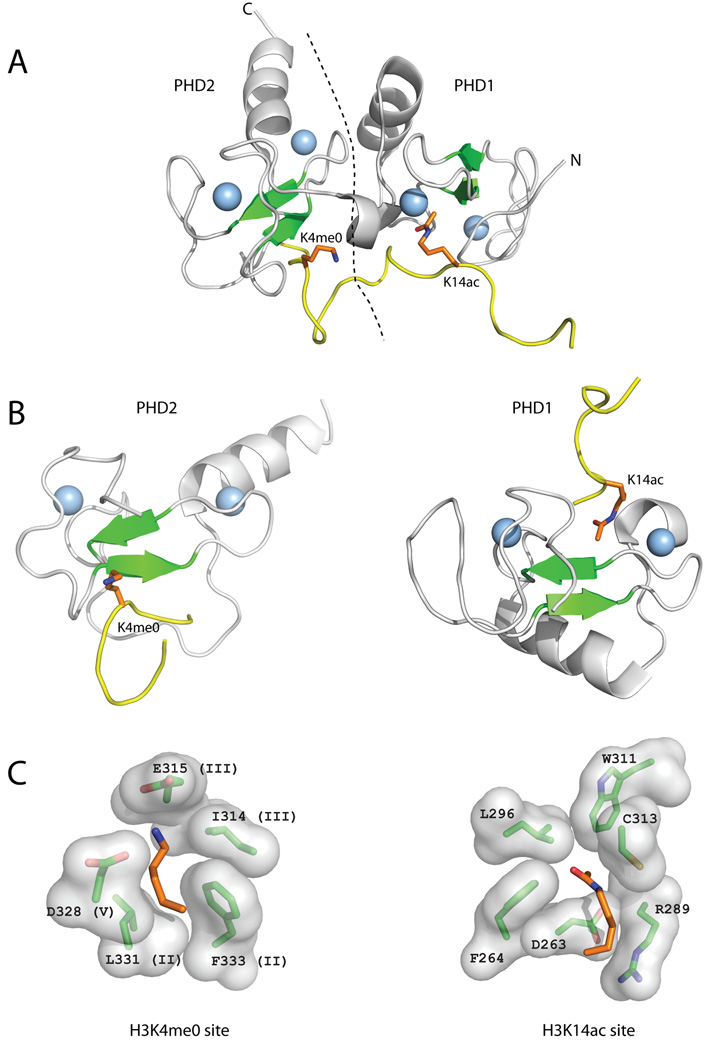
Figure 5. Reading of H3 tail by the DPF3b tandem PHD fingers.
The DPF3b tandem PHD fingers bind an H3 peptide acetylated on K14 (H3K14ac) and unmodified at R2 (H3R2me0) and K4 (H3K4me0) (A) The structure of the DPF3b tandem PHD fingers in complex with the histone H3 N-terminal tail peptide (PDB code 2kwj) is shown in cartoon representation (coloring as in Figure 2). The dotted line represents the boundary between PHD1 and PHD2. (B) The structures of PHD1 and PHD2 of DPF3b bound to H3K4me0 and H3K14ac, respectively, are shown separately in the same orientation and coloring scheme as the PHD fingers in Figure 2. (C) Close-up images of the H3K4me0 and H3K14ac recognition sites showing the residues involved in binding labeled according to residue number, and sequence region (for H3K4me3) (Figure 1).