Figure 2: The A-Rafshort protein does not interact with MST2.
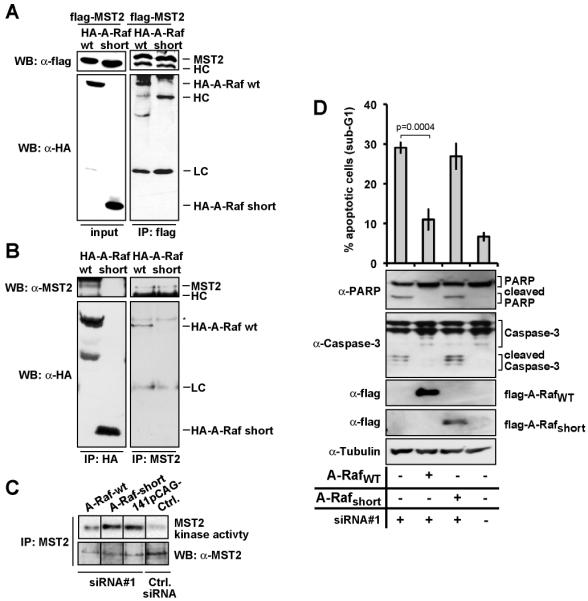
(A) Flag-tagged MST2 together with HA-tagged A-Rafwt or A-Rafshort, respectively, were expressed in HeLa cells. MST2 and A-Raf isoforms were immunoprecipitated (IP) using flag-tag specific antibodies. Flag-MST2 and A-Rafwt, but not the short isoform A-Rafshort, co-precipitated as shown by immunoblotting (WB). Shown are the representative results from three independent experiments. (B) HA-tagged A-Rafwt or A-Rafshort were expressed in HeLa cells. Endogenous MST2 and the HA-tagged A-Raf isoforms were immunoprecipitated (IP) with HA-tag or MST2 specific antibodies. MST2 and A-Rafwt, but not A-Rafshort, co-precipitated. Shown are the representative results from three independent experiments. (C) A-Rafwt but not A-Rafshort can suppress MST2 kinase activity. HeLa cells were transfected with hnRNP H (siRNA#1) and control siRNA. Where indicated, HA-A-Rafwt, HA-A-Rafshort, or control expression plasmids were co-transfected. MST2 kinase activity was assessed by in-gel kinase assays. (D) A-Rafwt but not A-Rafshort can suppress apoptosis induced by hnRNP H depletion. Apoptosis was determined in HeLa cells following transfection with hnRNP H-siRNA. Where indicated, cells were co-transfected with A-Rafwt, A-Rafshort expression plasmids. The data represent mean percentage of apoptosis with SD of three experiments. Additionally, cells were lysed, and expression of flag-tagged A-Raf, Caspase-3, and PARP was assessed by immunoblotting (lower panels).
