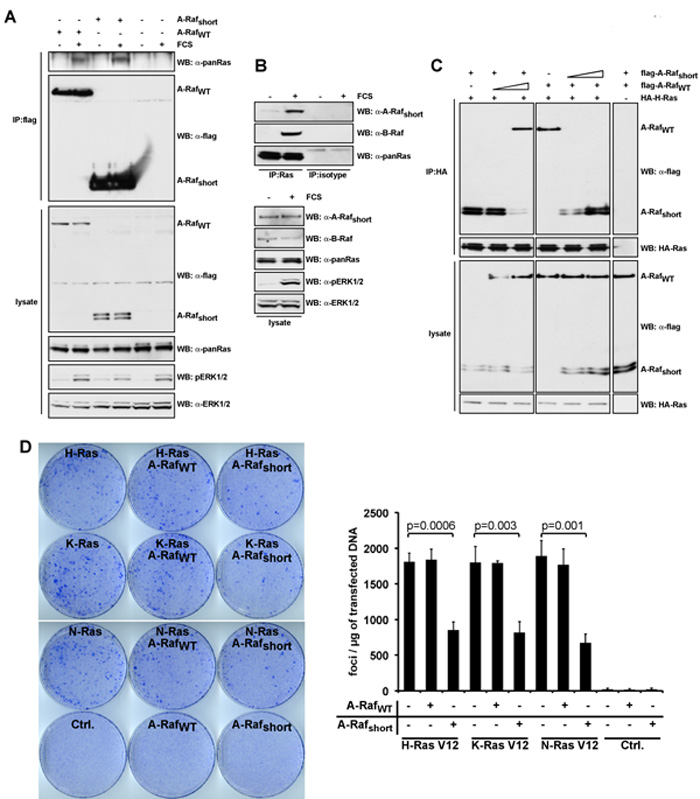
Figure 4: A-Rafshort interacts with activated Ras inhibiting Ras-ERK signalling.
(A) Flag-tagged A-Rafshort or A-RafWT were transfected in HeLa cells. Cells were serum-starved for 16 hours (0.1% FCS) or serum-stimulated (10% FCS) for 5min after starvation as indicated. A-Raf isoforms were immunoprecipitated with flag-tag specific antibodies, and analysed by immunoblotting for the interaction with Ras. (B) Endogenous Ras was immunoprecipitated from lysates of HeLa cells, which had been serum starved (0.1% FCS, 16 hours) or treated with full medium (10% FCS) after serum-starvation using a specifc antibody for Ras, and analysed by immunoblotting (upper panel). As an isotype control, an antibody specific for GFP was used (middle panel, ‘Isotype’). Lysates were immunoblotted for expression of A-Rafshort, Ras, B-Raf, phosphorylated ERK1/2, and ERK1/2 as loading control (lower panel). LC - light chain immunoglobulin. Shown are the representative results from three independent experiments. (C) A-Rafshort binds better to Ras. Either fixed or increasing amounts of Flag-tagged A-Rafshort or A-RafWT togther with HA-tagged H-Ras-V12 were transfected in HeLa cells as indicated. Cells were serum-starved for 16 hours (0.1% FCS) and serum-stimulated (10% FCS) for 5min after starvation. Ras complexes were immunoprecipitated, and analysed by immunoblotting for the A-Raf isoform interaction. (D) A-Rafshort inhibits Ras-induced cellular tranformation. NIH3T3 fibroblasts were tranfected with hyperactive Ras constructs. Cells were co-tranfected with either A-RafWT or A-Rafshort as indicated (left panel). Numbers indicate average number of foci per microgram of DNA (Ras constructs) (right panel). Error bars represent standard deviation of at least three independent experiments.