Abstract
We developed a novel ultrarapid immunohistochemical staining method in which an AC electric field is used to facilitate detection of tumor cells. Frozen sections of non-small cell lung cancer in lymph nodes were fixed in acetone for 2 min, after which they were incubated for 2 min with an anti-pancytokeratin antibody cocktail and then with EnVisionTM complex under an alternating current (AC) electric field. The sections were then incubated with a chromogen (3,3'diaminobenzidine) for 3 min and counterstained with hematoxylin. This method enabled detection of tumor cells in frozen sections in less than 15 min. In addition, we were able to reduce the amount of antibody used by more than 90% when the sections were incubated under the AC electric field for a longer period. This method could be a useful tool for frozen section diagnosis and research. Furthermore, with this method the cost of immunohistochemical staining can be reduced.
Keywords: immunohistochemical staining, lymph node metastases, micrometastases
I. Introduction
Detection of single tumor cells or small tumor cell clusters within lymph nodes and specimens from the surgical margin of resected tissue is very important in intraoperative frozen section analysis. In particular, micrometastases in lymph nodes sometimes cannot be detected on the basis of hematoxylin and eosin (HE) staining alone, but only after additional time-consuming immunohistochemical (IHC) investigation of paraffin-embedded tissue. This is noteworthy, as nodal micrometastasis is reportedly associated with poorer survival rates than node-negative disease in breast cancer [4, 22, 25] and non-small cell lung cancer (NSCLC) [5, 10]. Recently, the sentinel node concept was introduced into the surgical procedures for breast cancer [15], malignant melanoma [2], gastric cancer [14] and NSCLC [20]. The rapid, reliable and sensitive intraoperative histopathological evaluation of distant sentinel nodes represents a crucial element of successful application of the sentinel node concept. However, Turner et al. reported that 10 (14.3%) of 70 patients assessed as tumor-free using routine HE staining were found to be sentinel node-positive using IHC cytokeratin analysis [26]. Unfortunately, although the sensitivity of IHC analysis using anti-cytokeratin antibody is sufficient to detect micrometastasis, the standard protocol requires 2–4 hours to complete. To solve this problem, several investigators have proposed rapid methods that enable IHC protocols to be accomplished within only 12 to 30 min [6, 7, 12, 13, 16–19, 23]. In addition, we have developed a rapid method for detecting cytokeratin-positive tumor cells in lymph nodes using flow cytometry [24], which enables us to detect lymph node micrometastases within 40 min. None of these methods is problem free, however. For example, our use of flow cytometry was limited by the frequency of false-positives caused by the lack of morphological observation. To overcome that limitation, we have now developed a device that enables us to complete IHC analyses within 15 min using an alternating current (AC) electric field (patent pending). The aim of the present study was to evaluate the clinical significance, reliability, and sensitivity of the novel ultrarapid IHC method developed at our institute using this device.
II. Materials and Methods
Ten consecutive patients with NSCLC were enrolled in the study between July 2010 and August 2010 after obtaining signed informed consent. Surgically resected specimens were used under approval of the Institutional Review Boards at Akita University School of Medicine and University Hospital. After a preoperative evaluation, the patients were taken to an operating room, and the standard preparations were made for a thoracotomy, lung resection, and mediastinal lymph node dissection. Lymph nodes from each patient were used for this study.
IHC procedures
Tissue preparation
Lymph nodes were surgically resected from patients with NSCLC. Immediately after removal, the nodes were embedded in O.C.T. compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) and frozen for 30 sec in liquid acetone at −80°C in Histo-Tek Pino (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) and then transferred to a cryostat (CM1900 Leica, Wetzlar, Germany).
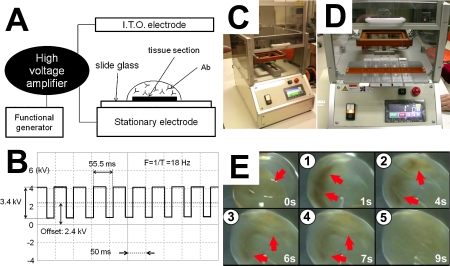
The device for IHC analysis (Fig. 1)
Fig. 1.
New device for AC electric field IHC. Panel A depicts a schematic diagram of a device for AC electric field IHC analysis. Panel B depicts a typical oscilloscope trace of the voltage and frequency of the AC electric field. A high voltage (3.4 kV, offset 2.4 kV), low frequency (18 Hz) AC electric field was applied to the sections. Panels 1C and 1D show the device, which was equipped with a humidifier to prevent evaporation of the antibody solution. To visualize the mixing effect of the device, the AC electric field was also applied to ferrite particles (average diameter: 50 nm) in PBS. Note that the brown particles (red arrows) were unevenly distributed at 1 and 4 sec, but were well mixed and homogeneously distributed within 9 sec (Panel E). I.T.O. electrode, indium tin oxide glass plate electrode; ab, antibody.
We have developed a device that reduces the time required for IHC, as well as the amount of antibody required for these analyses. With this device, we can apply a high-voltage, low-frequency AC electric field to the sections. The resultant coulomb force stirs the antibody solution on the sections. To examine the mixing effect of the device, the AC electric field was also applied to ferrite particles (average diameter: 50 nm) in PBS. To prevent evaporation of the antibody solution, the device was equipped with a humidifier, and all incubations were carried out under a humidified atmosphere. With the humidifier, the amount of antibody solution remained unchanged, even after 3 hr of incubation.
Rapid AC electric IHC procedure (Table 1)
Table 1.
Procedures and time for immunohistochemical staining
| Routine IHC procedure | Saving AC electric IHC procedure | Rapid AC electric IHC procedure | |
|---|---|---|---|
| Acetone fixation | 4°C | Room temperature | Room temperature |
| 10 min | 2 min | 2 min | |
| Washing with PBS | 5 min | 30 sec | 30 sec |
| 3 times | once | once | |
| Primary antibody | w/o AC electric field | AC electric field | AC electric field |
| 60 min | 60–180 min | 2 min | |
| Concentration of primary antibody | 5.0 µg/mL | 0.5–2.0 µg/mL | 5.0 µg/mL |
| (1:200) | (1:500–2,000) | (1:200) | |
| Washing with PBS | 5 min | 30 sec | 30 sec |
| 3 times | once | once | |
| EnVisionTM+ System/HRP Mouse | w/o AC electric field | AC electric field | AC electric field |
| 30 min | 2 min | 2 min | |
| Washing with PBS | 5 min | 30 sec | 30 sec |
| 3 times | once | once | |
| 3,3'diaminobenzidine | 3 min | 3 min | 3 min |
| Approximate time required | 148 min | 69–189 min | 11 min |
Serial frozen sections were cut at 5 µm and placed on slides, air-dried for 30 sec, fixed in acetone for 2 min at room temperature, air-dried for 15 sec at room temperature, and subjected to the staining procedure. No attempt was made to block endogenous peroxidase. For staining, the sections were incubated for 2 min with a monoclonal mouse anti-pancytokeratin antibody cocktail (AE1/AE3; PROGEN Biotechnik GmbH, Heidelberg, Germany) at 1:200 dilution (5 µg/mL) under a high-voltage (3.4 kV, offset 2.4 kV), low-frequency (18 Hz) AC electric field. The sections were then washed with PBS and incubated for 2 min with EnVisionTM+ System/HRP Mouse (DAB+) (Dako) under the same AC electric field. After again washing with PBS, the sections were developed with 3,3'diaminobenzidine substrate, counterstained with hematoxylin, dehydrated, and mounted on coverslips.
Standard IHC procedure (Table 1)
Serial frozen sections were cut at 5 µm and placed on slides, air-dried for 30 sec, fixed in acetone for 10 min at 4°C, air-dried for 15 sec at room temperature, and subjected to the staining procedure. No attempt was made to block endogenous peroxidase. For staining, the sections were first incubated for 60 min with AE1/AE3 at 1:200 dilution (5 µg/mL). They were then washed with PBS, incubated for 30 min with EnVisionTM+ System/HRP Mouse (DAB+), washed again with PBS, developed with 3,3'diaminobenzidine substrate, counterstained with hematoxylin, dehydrated, and mounted on coverslips.
Saving AC electric IHC procedure (Table 1)
Serial frozen sections were cut at 5 µm and placed on slides, air-dried for 30 sec, fixed in acetone for 2 min at room temperature, air-dried for 15 sec at room temperature, and subjected to the staining procedure. No attempt was made to block endogenous peroxidase. For staining, sections were incubated for 60–180 min with AE1/AE3 at 1:500–2,000 dilution (0.5–2.0 µg/mL) under a high voltage (3.4 kV, offset 2.4 kV), low frequency (18 Hz) AC electric field. The sections were then washed with PBS and incubated for 2 min with EnVisionTM+ System/HRP Mouse (DAB+) (Dako) under the same AC electric field. After washing again with PBS, the sections were developed with 3,3'diaminobenzidine substrate, counterstained with hematoxylin, dehydrated, and mounted on coverslips.
III. Results
We initially visualized the mixing effect of our device by applying the high voltage (3.4 kV), low frequency (18 Hz) AC electric field used for IHC analysis to ferrite particles in PBS. We observed that the ferrite particles were well mixed within 9 sec (Fig. 1E).
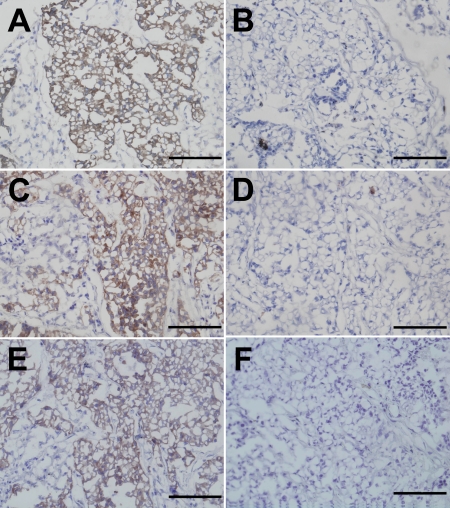
We then stained metastatic NSCLC cells in lymph nodes with an anti-pancytokeratin antibody cocktail, AE1/AE3, using standard IHC procedures or rapid AC electric or saving AC electric IHC procedures. Tumor cells within lymph nodes were stained equally well using the rapid AC electric procedure or the standard IHC procedure. Moreover, when the AC electric field was applied to sections for 60 min, the concentration of AE1/AE3 could be diluted 1:500, and when the AC electric field was applied for 180 min, the AE1/AE3 could be diluted 1:2,000. Without the AC electric field, the sections were not stained with AE1/AE3 at this dilution. Figure 2 depicts typical images obtained using the standard, rapid AC electric, and saving AC electric IHC procedures (dilution 1:2,000, 180 min AC electric field), respectively.
Fig. 2.
Pancytokeratin immunoreactivity toward metastatic NSCLC cells in a lymph node. The tissue sections were stained with anti-pancytokeratin antibody (AE1/AE3) using standard IHC (panel A), negative control for standard IHC (panel B), rapid AC electric field IHC (panel C), negative control for rapid AC electric field IHC (panel D), saving AC electric field IHC (dilution 1:2,000, 180 min AC electric field) (panel E), saving AC electric field IHC (panel F) procedures. Bar=100 µm.
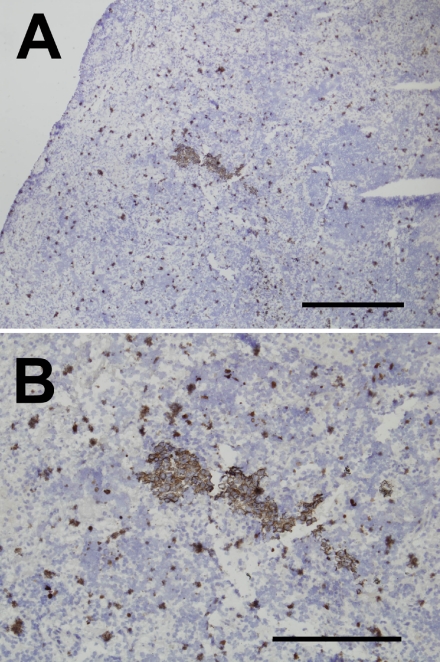
An essential purpose of IHC analysis during surgery for NSCLC is detection of sentinel node micrometastasis without misdiagnosis. This means that to apply the rapid AC electric field IHC procedure in practice, its ability to detect micrometastases must be at least equal to that of the standard IHC procedure. Therefore, to further determine the effectiveness of the AC electric field on the rapid IHC procedure, we compared the abilities of the standard and rapid AC electric field IHC procedures to detect lymph node micrometastases. Typical images of lymph node micrometastasis obtained using the rapid AC electric field IHC procedure are shown in Figure 3. Endogenous peroxidase-positive granulocytes were also observed around cytokeratin-positive cancer cell nests. However, the cancer cells could be distinguished from endogenous peroxidase-positive leukocytes based on their morphology. Therefore, to shorten the staining period, we did not use endogenous peroxidase blockage in the present study. Note that the lymph node micrometastases detected using the standard IHC procedure were always detectable using the rapid AC electric field IHC procedure.
Fig. 3.
Detection of micrometastasis using the rapid AC electric field IHC procedures. To confirm the ability of the rapid AC electric field IHC procedure to detect lymph node micrometastasis, we immunohistochemically stained lymph nodes positive for NSCLC micrometastasis with anti-pancytokeratin antibody (AE1/AE3). Panels A and B show low and high magnification images using the rapid AC electric field IHC procedures, respectively. Endogenous peroxidase-positive granulocytes were often observed. However, cytokeratin-positive cancer cell nests could be readily discerned based on their morphology. Bars=1,000 µm (panel A), 500 µm (panel B).
IV. Discussion
In the present study we demonstrated that tumor cells in lymph nodes can be detected within 11 min under a high voltage, low frequency AC electric field using the device developed at our institute. Furthermore, not only does this method enable rapid IHC staining, it can also potentially reduce the cost of the IHC procedure.
Conventional analysis of frozen tissue sections using HE staining enables diagnosis within 20 min at most institutions [27], but the sensitivity of this method for detecting micrometastases is comparatively low. By contrast, the sensitivity of IHC analysis using anti-cytokeratin antibody is sufficient to detect micrometastasis, but the standard IHC procedure commonly requires 2–4 hr to complete. To address this issue, several investigators proposed rapid methods that enable the IHC procedure to be accomplished within only 12 to 30 min [6, 7, 13, 16–18, 23, 24, 27]; however, these methods are not problem-free. For example, the new EnVisionTM system has been described as a very sensitive and rapid detection system for IHC [24, 27], and Kämmerer et al. and Mönig et al. reduced the time for immunostaining of frozen sections to less than 13 min using the EnVision system. To do so, however, they applied a higher concentration of primary antibody than is used for standard IHC [13, 17]. By contrast, our ultrarapid AC electric field IHC procedure required about the same amount of time as the EnVisionTM system, and we were able to use the same concentration of primary antibody used in the standard IHC procedure. Given the expense of primary antibodies, we believe this represents a significant advantage over other procedures. It is also known that microwave irradiation shortens IHC times. Hatta et al. reported that IHC could be completed within 15 min using an intermittent microwave irradiation method in combination with a prepared immune complex consisting of the primary antibody, secondary antibody and EnVisionTM-solution [11]. The utility of our AC electric field method is comparable to this microwave approach.
The cost of IHC analysis is high because primary antibodies are expensive. Therefore, reducing the amount of primary antibody needed for IHC can have a significant impact on cost. In particular, diagnosis of lymph node micrometastasis requires the use of step sections of the lymph nodes in question. According to the 6th edition of cancer staging manual of the American Joint Committee on Cancer, micrometastases are defined as being >0.2 mm but ≤2.0 mm in diameter [9]. Consequently, step sections must be collected every 0.2 mm so as not to miss any tumor tissue. In an effort to reduce the amount of primary antibody needed, we applied the AC electric field IHC procedure. We found that when the AC electric field was applied to sections for 180 min, the concentration of primary antibody could be reduced by more than 90%.
The mechanism by which our method promotes the antigen-antibody reaction is not fully understood. In this study we applied a high voltage (3.4 kV, offset 2.4 kV), low frequency (18 Hz) AC electric field to IHC. Before we adopted this approach, we tested a variety of other methods for stirring small amounts of solution, ultimately determining that application of an AC electric field worked best under our conditions. It is known that when an AC electric field is applied to a solution, electro-osmotic vortices are produced [3], which were used by Wang et al. [28] to induce non-contact mixing. However, we do not believe such vortices are responsible for the enhanced antigen-antibody reaction seen in the present study. We previously showed that an interaction force affecting a droplet can be generated as a coulomb force applied from an AC electric field [1]. The generated force reflects the difference between dielectric constants and the electric conductivity. In particular, when a droplet is attracted to the upper electrode by giving the electric field a positive polarity, a wave is generated, and the stirring phenomenon is caused by changes in the surface of this wave. We speculate that this stirring promotes the antigen-antibody reaction.
Although the main purpose of the ultra-rapid IHC is the diagnosis of sentinel node micrometastasis during the surgery, avoiding axillary lymph node dissection based on the results of sentinel node biopsy is now controversial in breast cancer. Recently, the American College of Surgeons Oncology Group Z0011 trial demonstrated that, among patients with limited sentinel node metastatic breast cancer, the use of sentinel node dissection alone did not result in inferior survival, as compared to axillary lymph node dissection [8]. This result indicates that intraoperative diagnosis of sentinel node micrometastasis may not be required, at least in breast cancer. We speculate that the reason for this is that radiation, chemotherapy, and endocrine therapy are more effective with breast cancer than with cancers in other organs. Unfortunately, radiation and chemotherapy are not particularly effective in NSCLC. In addition, Ou and Zell demonstrated that the number of lymph nodes removed at surgery in patients without apparent lymph node metastasis is a significant prognostic factor in NSCLC [21]. Furthermore, mediastinal lymph node dissection is still the standard surgical procedure for patients with NSCLC. Therefore, lymph node micrometastasis should not be ignored when applying sentinel node mapping to NSCLC.
In conclusion, we were able to demonstrate the usefulness of our new method of AC electric field IHC analysis. However, the method was only tested using a single antibody in a limited number of experiments. Further investigation using other antibodies in larger clinical studies will be needed to confirm the utility of this method.
V. Conflict of interest statement
Hiroshi Toda and other co-authors have no conflict of interest.
VI. References
- 1.Akagami Y., Asari K., Jeyadevan B., Fujita T., Umehara N. ER fluid finishing using rotating electrode. J. Intel. Mat. Syst. Str. 1999;10:753–756. [Google Scholar]
- 2.Amersi F., Morton D. L. The role of sentinel lymph node biopsy in the management of melanoma. Adv. Surg. 2007;41:241–256. doi: 10.1016/j.yasu.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Y., Chang H. C. Nonlinear Smoluchowski slip velocity and micro-vortex generation. J. Fluid. Mech. 2002;461:229–238. [Google Scholar]
- 4.Chen S. L., Hoehne F. M., Giuliano A. E. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann. Surg. Oncol. 2007;214:3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 5.Coello M. C., Luketich J. D., Litle V. R., Godfrey T. E. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin. Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Dabbs D. J., Hafer L., Abendroth C. S. Intraoperative immunocytochemistry of cytologic scrape specimens. A rapid immunoperoxidase method for triage and diagnosis. Acta Cytol. 1995;39:157–163. [PubMed] [Google Scholar]
- 7.Eudy G. E., Carlson G. W., Murray D. R., Waldrop S. M., Lawson D., Cohen C. Rapid immunohistochemistry of sentinel lymph nodes for metastatic melanoma. Hum. Pathol. 2003;34:797–802. doi: 10.1016/s0046-8177(03)00290-9. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano A. E., Hunt K. K., Ballman K. V., Beitsch P. D., Whitworth P. W., Blumencranz P. W., Leitch A. M., Saha S., McCall L. M., Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene F. L., Page D. L., Fleming I. D., et al. (eds) 6th ed., Springer-Verlag; New York, NY: 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 10.Gu C. D., Osaki T., Oyama T., Inoue M., Kodate M., Dobashi K., Oka T., Yasumoto K. Detection of micrometastatic tumor cells in pN0 lymph nodes of patients with completely resected nonsmall cell lung cancer: impact on recurrence and survival. Ann. Surg. 2002;235:133–139. doi: 10.1097/00000658-200201000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatta H., Tsuneyama K., Kumada T., Zheng H., Cheng C., Cui Z., Takahashi H., Nomoto K., Murai Y., Takano Y. Freshly prepared immune complexes with intermittent microwave irradiation result in rapid and high-quality immunostaining. Pathol. Res. Pract. 2006;202:439–445. doi: 10.1016/j.prp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ito M., Minamiya Y., Kawai H., Saito S., Saito H., Imai K., Ogawa J. Intraoperative detection of lymph node micrometastasis with flow cytometry in non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2005;130:753–758. doi: 10.1016/j.jtcvs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Kämmerer U., Kapp M., Gassel A. M., Richter T., Tank C., Dietl J., Ruck P. A new rapid immunohistochemical staining technique using the EnVision antibody complex. J. Histochem. Cytochem. 2001;49:623–630. doi: 10.1177/002215540104900509. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa Y., Saha S., Kubo A., Kitajima M. Sentinel node for gastrointestinal malignancies. Surg. Oncol. Clin. N Am. 2007;16:71–80. doi: 10.1016/j.soc.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Mabry H., Giuliano A. E. Sentinel node mapping for breast cancer: progress to date and prospects for the future. Surg. Oncol. Clin. N Am. 2007;16:55–70. doi: 10.1016/j.soc.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M., Natsugoe S., Ishigami S., Uenosono Y., Takao S., Aikou T. Rapid immunohistochemical detection of lymph node micrometastasis during operation for upper gastrointestinal carcinoma. Br. J. Surg. 2003;90:563–566. doi: 10.1002/bjs.4083. [DOI] [PubMed] [Google Scholar]
- 17.Mönig S. P., Luebke T., Soheili A., Landsberg S., Dienes H. P., Hölscher A. H., Baldus S. E. Rapid immunohistochemical detection of tumor cells in gastric carcinoma. Oncol. Rep. 2006;16:1143–1147. [PubMed] [Google Scholar]
- 18.Nährig J. M., Richter T., Kuhn W., Avril N., Flatau B., Kowolik J., Höfler H., Werner M. Intraoperative examination of sentinel lymph nodes by ultrarapid immunohistochemistry. Breast J. 2003;9:277–281. doi: 10.1046/j.1524-4741.2003.09405.x. [DOI] [PubMed] [Google Scholar]
- 19.Novis D. A., Zarbo R. J. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 32868 frozen sections in 700 hospitals. Arch. Pathol. Lab. Med. 1997;121:559–567. [PubMed] [Google Scholar]
- 20.Ono T., Minamiya Y., Ito M., Saito H., Motoyama S., Nanjo H., Ogawa J. Sentinel node mapping and micrometastasis in patients with clinical stage IA non-small cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 2009;9:659–661. doi: 10.1510/icvts.2009.214197. [DOI] [PubMed] [Google Scholar]
- 21.Ou S. H., Zell J. A. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J. Thorac. Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 22.Park D., Kåresen R., Naume B., Synnestvedt M., Beraki E., Sauer T. The prognostic impact of occult nodal metastasis in early breast carcinoma. Breast Cancer Res. Treat. 2009;118:57–66. doi: 10.1007/s10549-009-0340-2. [DOI] [PubMed] [Google Scholar]
- 23.Richter T., Nahrig J., Komminoth P., Kowolik J., Werner M. Protocol for ultrarapid immunostaining of frozen sections. J. Clin. Pathol. 1999;52:461–463. doi: 10.1136/jcp.52.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabattini E., Bisgaard K., Ascani S., Poggi S., Piccioli M., Ceccarelli C., Pieri F., Fraternali-Orcioni G., Pileri S. A. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J. Clin. Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truong P. T., Vinh-Hung V., Cserni G., Woodward W. A., Tai P., Vlastos G. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur. J. Cancer. 2008;44:1670–1677. doi: 10.1016/j.ejca.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Turner R. R., Ollila D. W., Krasne D. L., Giuliano A. E. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann. Surg. 1997;226:271–278. doi: 10.1097/00000658-199709000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyberg M., Horn T., Francis D., Askaa J. Immunohistochemical identification of neuron-specific enolase, synaptophysin, chromogranin and endocrine granule constituent in neuroendocrine tumours. Acta Histochem. Suppl. 1990;38:179–181. [PubMed] [Google Scholar]
- 28.Wang S. C., Chen H. P., Lee C. Y., Yu C. C., Chang H. C. AC electro-osmotic mixing induced by non-contact external electrodes. Biosens. Bioelectron. 2006;22:563–567. doi: 10.1016/j.bios.2006.05.032. [DOI] [PubMed] [Google Scholar]