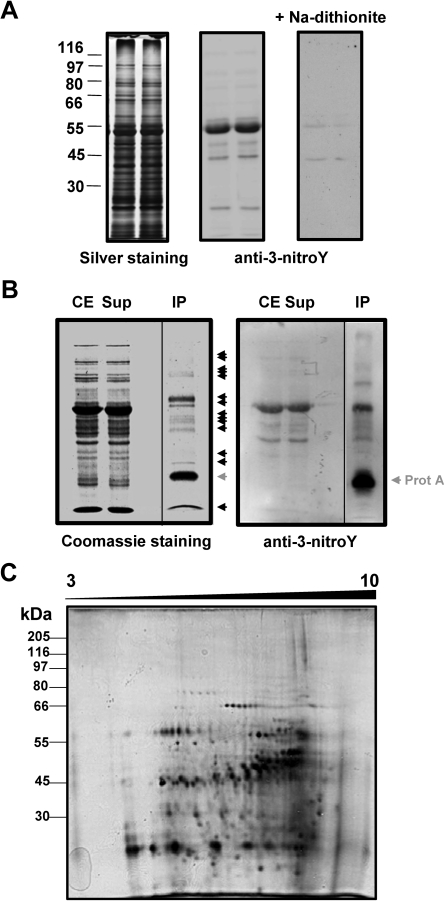
Fig. 1.
Detection of 3-nitroY-containing proteins. (A) Crude protein extracts (10 μg per lane) were separated using 10% SDS–PAGE in duplicate. The left panel shows the silver-stained gel with the position of a molecular weight protein ladder. The central panel shows the corresponding western blot performed with anti-3-nitroY primary antibody, and the right panel the corresponding western blot after reduction of 3-nitroY to 3-aminoY with 100 mM sodium dithionite for 30 min. (B) In vivo immunoprecipitation of Arabidopsis 3-nitroY-containing proteins. Crude extracts (CE) were immunoprecipitated with antibody against 3-nitroY. The resulting supernatants (Sup) and immunoprecipitated proteins (IP) alongside the CE were separated by one-dimensional SDS–PAGE in duplicate and either Coomassie stained (left panel) or transferred to a nitrocellulose membrane and probed with anti-3-nitroY antibodies by western blot (right panel). Immunoprecipitated proteins detected by one-dimensional SDS–PAGE are marked with black arrowheads. The protein A which is released from the resin in the immunoprecipitates is marked with a grey arrowhead. (C) Immunoprecipitated proteins (0.1 mg) were separated by 2-DE with isoelectric focusing in the range of pH 3–10 NL and a second dimension 10% gel. The identification of spots corresponding to nitrated proteins was performed by comparing four independent sets of 2-DE gels corresponding to biologically independent replicates with similar spot patterns. Molecular mass marker positions are indicated in kDa on the left side. Proteins were silver stained.