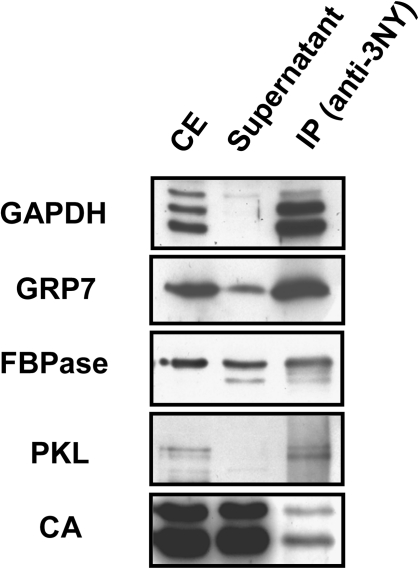
Fig. 2.
Confirmation of the presence of proteins identified through shotgun proteomic analysis in the immnunopurified nitroproteome. Crude protein extracts (CE) were immunoprecipitated with anti-3-nitroY (anti-3-NY) antibodies. The CE, supernatant, and immunoprecipitate (IP) were separated by 12% SDS–PAGE, transferred to a nitrocellulose membrane, and probed with specific antibodies raised against chloroplastic glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glycine-rich protein 7 (GRP7), fructose bisphosphatase (FBPase), PICKEL (PKL), or carbonic anhydrase (CA). The procedure started from 1mg of total protein in the crude extract that was immunoprecipitated as described in the Materials and methods, and then the whole IP was loaded on the gel along with 1% of the CE input and the corresponding supernatant.