Abstract
Nutritional ecological theory predicts that predators should adjust prey capture and consumption rates depending on the prey's nutritional composition. This would affect the predator's functional response, at least at high prey densities, i.e. near predator satiation. Using a simple fruitfly-wolf spider laboratory system in Petri dishes, we found that functional responses changed from day to day over a 7 day period. After 1 to 2 days of feeding, dome-shaped functional responses (i.e. reduced predation at highest prey densities) appeared in spiders fed nutritionally imbalanced prey, compared with steadily increasing or asymptotic functional responses with nutritionally near-optimal prey. Later again (days 5–7), the difference disappeared as the level of the functional response was reduced in both treatments. Experiments with adult females in spring and subadult spiders in autumn revealed opposite patterns: a dome-shaped response with high-lipid prey for reproductive females, for which protein-rich prey are optimal, and a dome-shaped (or simply reduced) response with high-protein prey for pre-winter subadults, for which high-lipid flies are the optimal prey. Our results have implications for predation theory and models of biological control that have, so far, neglected nutritional aspects; in particular, the dynamic nutritional state of the predators should be incorporated.
Keywords: Araneae, biological control, geometric framework, Lycosidae, predation, nutrient composition
1. Introduction
The functional response denotes the relationship between a predator's killing or consumption rate and the density of the prey population in the environment. Three types of functional responses have been recognized (Holling's type I–III; [1–3]). In the standard versions, all three types increase to a maximal or asymptotic value that represents the maximal consumption or prey handling capacity of the predator. However, all three types may show a lowered functional response at very high prey densities, i.e. above a certain prey density, predator success is reduced, leading to dome-shaped functional response curves [4]. Since Holling [1,2], this has been ascribed to predators becoming confused at high prey densities, or to prey chemical defences reaching deterrent or toxic levels when consumed in large amounts [4–7]. We here identify a novel mechanism that may lead to a dome-shaped functional response in a predator, viz. an imbalanced nutrient composition of the prey. Our study provides an empirical example of how population-level phenomena can be predicted from nutritional ecology via optimal foraging theory [8–10].
The capture and consumption rate of a consumer in relation to the nutritional composition of the food can be predicted from the nutritionally explicit theory of optimal foraging [11] based on the geometric framework [11–13], more precisely from the fitness landscape and corresponding compromise rules for intake of the considered nutrients. Increased, unchanged or lowered predation rates may result when the prey deviate from the optimal nutrient composition, depending on which rule is followed. The predators analysed so far all show asymmetric responses, depending on whether the imbalance is towards the protein or the lipid side of the optimal composition [14,15]. Predators tend to exaggerate intake of protein-rich foods but maintain or even lower the intake of lipid-rich foods compared with balanced food. We therefore expect an exaggerated functional response towards protein-biased prey and a reduced functional response towards lipid-biased prey, at high prey densities. However, if the optimal intake composition lies towards the high-lipid side, as may be the case when the animals prepare for hibernation, these predictions may be modified or even turned around. We used wolf spiders in two different phases of the life cycle with widely divergent nutritional demands, for testing the effects of prey nutrient composition on the shape of the functional response. Since predator satiation and nutritional balance change continuously as a result of previous feeding, and thus affect the predator's current demands, the numerical level of the functional responses will be lowered and the effects of nutritional balance on the shape of the response should change over time. We therefore repeated the experiments daily over 7 days, mimicking the situation of a prey species that emerges at a certain season and is active in the habitat for a period of several days.
2. Material and methods
The experimental system consisted of wolf spiders Pardosa amentata as the predator and wild-type fruitflies Drosophila melanogaster as prey. Protein-rich and lipid-rich flies (lipid : protein ratios 0.10 and 0.89; see the electronic supplementary material) were offered to groups of spiders as pre-treatments and treatments in the functional response experiments in a factorial design. Two experiments were completed, one with adult females, and the other with subadult spiders. For the first experiment, we used adults that reproduce in early spring. The spiders hibernate as subadults and were collected for the second experiment in that stage in September. The experiments were run in Petri dishes with spiders offered one of four densities of fruitflies. For details of the set-up, experimental procedures and data analysis, see the electronic supplementary material.
3. Results
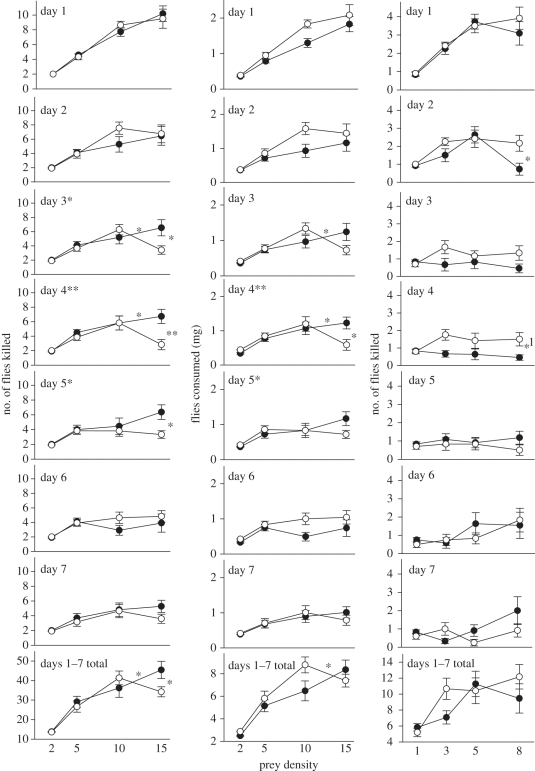
In the repeated-measures analysis of all the data from each experiment (electronic supplementary material, table S1), there were main effects of prey density and spider mass, and interaction effects of days with both fly type and prey density. There were no effects of pre-treatments. In the analyses of each day's results, there were only two weakly significant interactions involving pre-treatment (electronic supplementary material, table S2). Therefore, pre-treatments are combined in the graphical presentations (figure 1). The days-interactions reflect systematic changes in the level of the functional responses and the shapes of the curves over the 7 day periods. Responses were strongest on the first day and reduced over subsequent days. The changes in shape of the response curves differed between experimental fly treatments. In the series with adult spiders (figure 1 left column), both treatments initially showed increasing functional response, with no differences between treatments. However, from day 3 through to day 5, killing rates were reduced in the spiders offered the highest densities of high-lipid flies, while still increasing for those offered high-protein flies. These differences are indicated both by significant fly-type × prey-density interactions, and by significant differences between treatments at the highest prey density (figure 1 and electronic supplementary material, table S2). On days 6 and 7, the functional responses for both fly types had turned into a simple type II at a low total response level. The differences during days 3–5 were strong enough that the cumulated dataset showed a significant difference in killing rate between fly types at the highest fly density (bottom panel). This pattern was paralleled in the corresponding consumption rates (figure 1 middle column), although significance levels were generally lower. In the experiment with subadults (figure 1 right column), the overall pattern is similar, except that fewer dates show significant differences between fly types at the highest density, and there are no fully significant prey-density × fly-type interactions. There are, however, stronger indications of differences in capture rates between fly types even at lower fly densities (days 3 and 4, electronic supplementary material, table S2). Furthermore, it is noted that the direction of difference between fly types is opposite to that in the adult experiment: capture rates were reduced in the groups offered high-protein flies compared with those offered high-lipid flies.
Figure 1.
Number of fruitflies killed (left column) and dry mass of flies consumed (middle column) (mean ± s.e.) by adult females, and number of fruitflies killed by subadults (right column) of the wolf spider Pardosa amentata during each of 7 days, and cumulated for the 7 days, in relation to prey density in experimental Petri dishes. Asterisks after the day number indicate a significant prey-density × fly-type interaction in ANOVA covering all four prey densities; asterisks between the two highest densities indicate a significant prey-density × fly-type interaction in ANOVA covering only those two densities; asterisks to the right of the curves indicate significant difference between fly-type treatments at the highest prey density in t-tests (1Welch t-test used owing to unequal variances). *p < 0.05, **p < 0.01. Detailed statistical tables in the electronic supplementary material, tables S1 and S2.
4. Discussion
The experiments gave three striking results: (i) the functional response changed over time both with respect to its magnitude and shape; (ii) prey nutrient composition significantly affected the shape of the functional response curves on some dates; but (iii) the direction of the effect was opposite in the two experiments.
Although the spiders were only mildly starved at the start of the tests, they killed more flies on the first day and killing rate more or less gradually decreased over the 7 day period. This reveals a simple satiation effect. The difference in shape of the functional response curves in the adult experiment during days 3–5 (and for the whole experimental period) is the one expected for a predator with an intermediate or protein-biased optimal lipid : protein ratio: a simple type II response with balanced prey and a dome-shaped response with imbalanced prey. The delayed response is also what is expected owing to a nutrient specific ‘satiation effect’ resulting from increasing nutritional imbalance of the spiders the more of the lipid-rich flies were consumed [16]. The pre-treatments were designed to create this effect from the start, but they may have been too short or their effect may initially have been overridden by hunger effects. The single significant pre-treatment × experimental fly-type interaction appeared on day 4 when the nutritional effect was strongest (electronic supplementary material, table S2).
The fitness landscape for the wolf spiders is imperfectly known. However, P. amentata juveniles had maximal growth rate and minimal development time when fed flies with a lipid : protein ratio of 0.15–0.25 [17], i.e. at a lipid : protein ratio close to the highly protein-biased food used in the present study. Most insects show a high demand for protein during oogenesis [18]; another generalist predator, the carabid beetle Anchonemus dorsalis thus maximizes its fecundity at a lipid : protein ratio of 0.36 (K. Jensen, D. Mayntz, S. Toft, D. Raubenheimer & S. J. Simpson 2011, unpublished data). It is therefore likely that our protein-rich flies are close to the optimal lipid : protein ratio also for the reproductive females, though probably more protein-rich than optimal. The lipid-rich flies, on the other hand, were far removed from the optimal nutrient composition and biased to the lipid side. On days 6 and 7, the response curves converge again owing to increasing satiation also in the spiders offered near-optimal prey. The spiders offered the lipid-rich flies did not further reduce their capture rate on these days, probably because they had previously been feeding at reduced rates and thus were not satiated to the same extent as the spiders offered protein-rich flies.
In the experiment with subadult spiders, it was the curve for protein-rich flies that was dome-shaped or reduced, and the one for lipid-rich flies of a simple type II. Subadult spiders were collected in autumn when the spiders were preparing for hibernation. Spiders, as most other temperate zone animals, prepare for winter by accumulating fat reserves [19] and thus should have a high demand for lipid-rich food. The carabid A. dorsalis show self-selection of semi-artificial diets with a lipid : protein ratio of ca 0.7 before hibernation (S. Toft, N. Noreika, N. Escobedo, K. Jensen & D. Mayntz 2011, unpublished data) and 0.6 just after hibernation [14], reaching 0.36 during reproduction (K. Jensen, D. Mayntz, S. Toft, D. Raubenheimer & S. J. Simpson 2011, unpublished data). We expect a similar contrast in lipid demand between autumn and spring for the wolf spiders. These results make it likely that the lipid-rich flies are near-optimal for the subadult spiders in autumn, and consequently, that the protein-rich flies are the imbalanced prey. The opposite response patterns are thus a result of different nutritional requirements in different phases of the life cycle.
Prey nutrient quality affects predator fitness [20,21], and through aggregative and reproductive numerical responses as well as the functional response must influence the overall ability of predators to control their prey [3]. Our results thus have implications for the theory of predation and biological control that has so far completely neglected aspects of prey nutritional quality. Our results indicate that a predator population feeding on a single abundant prey species over a period (e.g. a pest in outbreak) will show a prey-specific sequence of responses, because individual behaviour changes over time owing to changing physiological states (satiation level, nutritional balance). The predators' dynamic physiological states should be explicitly included in predation models to make them more realistic.
Acknowledgements
S.T. was supported by grants from the Danish Research Council (FNU) and the Carlsberg Foundation. We are indebted to David Raubenheimer and an anonymous reviewer for suggestions that greatly improved the article.
References
- 1.Holling C. S. 1961. Principles of insect predation. Annu. Rev. Entomol. 6, 163–182 10.1146/annurev.en.06.010161.001115 (doi:10.1146/annurev.en.06.010161.001115) [DOI] [Google Scholar]
- 2.Holling C. S. 1965. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 45, 1–60 [Google Scholar]
- 3.Hassell M. H. 1978. The dynamics of arthropod predator–prey systems. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 4.Jeschke J. M., Kopp M., Tollrian R. 2004. Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol. Rev. 79, 337–349 10.1017/S1464793103006286 (doi:10.1017/S1464793103006286) [DOI] [PubMed] [Google Scholar]
- 5.Young S., Watt P. J., Grover J. P., Thomas D. 1994. The unselfish swarm? J. Anim. Ecol. 63, 611–618 10.2307/5227 (doi:10.2307/5227) [DOI] [Google Scholar]
- 6.Watt P. J., Chapman R. 1998. Whirligig beetle aggregations: what are the costs and the benefits? Behav. Ecol. Sociobiol. 42, 179–184 10.1007/s002650050429 (doi:10.1007/s002650050429) [DOI] [Google Scholar]
- 7.Jeschke J. M., Tollrian R. 2005. Effects of predator confusion on functional responses. Oikos 111, 547–555 10.1111/j.1600-0706.2005.14118.x (doi:10.1111/j.1600-0706.2005.14118.x) [DOI] [Google Scholar]
- 8.McGill B. J., Enquist B. J., Weiher E., Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 10.1016/j.tree.2006.02.002 (doi:10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 9.Raubenheimer D., Simpson S. J., Mayntz D. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16 10.1111/j.1365-2435.2009.01522.x (doi:10.1111/j.1365-2435.2009.01522.x) [DOI] [Google Scholar]
- 10.Simpson S. J., Raubenheimer D., Charleston M. A., Clissold F. J., the ARC-NZ Vegetation Function Network Herbivory Working Group 2009. Modelling nutritional interactions: from individuals to communities. Trends Ecol. Evol. 25, 53–60 10.1016/j.tree.2009.06.012 (doi:10.1016/j.tree.2009.06.012) [DOI] [PubMed] [Google Scholar]
- 11.Simpson S. J., Sibly R. M., Lee K. P., Behmer S. T., Raubenheimer D. 2004. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1311 10.1016/j.anbehav.2004.03.003 (doi:10.1016/j.anbehav.2004.03.003) [DOI] [Google Scholar]
- 12.Raubenheimer D., Simpson S. J. 1997. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10, 151–179 10.1079/NRR19970009 (doi:10.1079/NRR19970009) [DOI] [PubMed] [Google Scholar]
- 13.Behmer S. T. 2009. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 54, 165–187 10.1146/annurev.ento.54.110807.090537 (doi:10.1146/annurev.ento.54.110807.090537) [DOI] [PubMed] [Google Scholar]
- 14.Raubenheimer D., Mayntz D., Simpson S. J., Toft S. 2007. Nutrient-specific compensation following diapause in a predator: implications for intraguild predation. Ecology 88, 2598–2608 10.1890/07-0012.1 (doi:10.1890/07-0012.1) [DOI] [PubMed] [Google Scholar]
- 15.Mayntz D., Nielsen V. H., Sørensen A., Toft S., Raubenheimer D., Hejlesen C., Simpson S. J. 2009. Balancing of protein and lipid intake by a mammalian carnivore, the mink, Mustela vison. Anim. Behav. 77, 349–355 10.1016/j.anbehav.2008.09.036 (doi:10.1016/j.anbehav.2008.09.036) [DOI] [Google Scholar]
- 16.Mayntz D., Raubenheimer D., Salomon M., Toft S., Simpson S. J. 2005. Nutrient-specific foraging in invertebrate predators. Science 307, 111–113 10.1126/science.1105493 (doi:10.1126/science.1105493) [DOI] [PubMed] [Google Scholar]
- 17.Jensen K., Mayntz D., Toft S., Raubenheimer D., Simpson S. J. 2011. Prey nutrient composition has different effects on Pardosa wolf spiders with dissimilar life histories. Oecologia 165, 577–583 10.1007/s00442-010-1811-1 (doi:10.1007/s00442-010-1811-1) [DOI] [PubMed] [Google Scholar]
- 18.Wheeler D. 1996. The role of nourishment in oogenesis. Annu. Rev. Entomol. 41, 407–431 10.1146/annurev.en.41.010196.002203 (doi:10.1146/annurev.en.41.010196.002203) [DOI] [PubMed] [Google Scholar]
- 19.Collatz K., Mommsen T. 1974. Life cycle and annual variations in body constituents of the spider Tegenaria atrica C. L. Koch (Agelenidae). J. Comp. Physiol. 91, 91–109 10.1007/BF00696158 (doi:10.1007/BF00696158) [DOI] [PubMed] [Google Scholar]
- 20.Toft S. 1995. Value of the aphid Rhopalosiphum padi as food for cereal spiders. J. Appl. Ecol. 32, 552–560 10.2307/2404652 (doi:10.2307/2404652) [DOI] [Google Scholar]
- 21.Toft S., Wise D. H. 1999. Growth, development, and survival of a generalist predator fed single- and mixed-species diets of different quality. Oecologia 119, 191–197 10.1007/s004420050776 (doi:10.1007/s004420050776) [DOI] [PubMed] [Google Scholar]