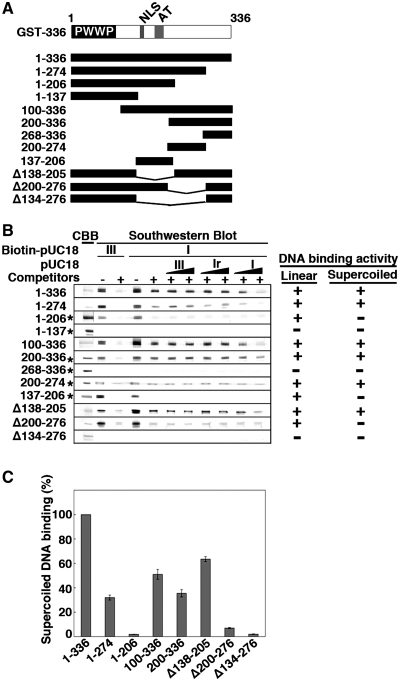
Figure 3.
Nailing down the region of SBP75/LEDGF that is responsible for supercoiled DNA binding activity by Southwestern blotting. (A) Deletion mutants of GST-SBP75/LEDGF used for the analysis are illustrated. Domain structure of N-terminal fragment (amino acids 1–336) is shown on the top. (B) Protein amounts loaded to the gel were either 5 µg (indicated by asterisks on the left) or 1.5 µg (others). Blotted membranes were incubated with biotin-labeled pUC18 as in Figure 1A. DNA binding to linear DNA or preferential binding to supercoiled DNA is summarized on the right. (C) Relative binding activity of SBP75/LEDGF deletion mutants. GST-fusion proteins (50 ng each, except GST-1–206 and GST-200–336 that were100 ng) were loaded to 7.5% polyacrylamide gel. Binding of biotin-labeled pUC18 form I DNA in the presence of non-specific competitors was measured by scanning densitometry. To express the binding activity on a molar basis with respect to proteins, an identical blot was probed with affinity-purified anti-GST polyclonal antibody and the quantified band intensities were used to normalize the DNA binding. Data shown are relative values to GST-1–336 (average for three independent experiments with error bars).