Abstract
Salmonella typhimurium survives within macrophages and causes a fatal infection in susceptible strains of mice. A number of S. typhimurium mutants that contain Tn10 insertions in genes which are necessary for survival within the macrophage have been isolated. To demonstrate the importance of each gene in intracellular survival, the mutations were transduced into a smooth-strain background and the ability to survive intracellularly was assayed in five different populations of macrophages. The majority of the original macrophage-sensitive mutants retained the macrophage-sensitive phenotype in the smooth-strain background. The ability to survive or grow within macrophages varied with both the source of macrophages and the individual mutants. S. typhimurium grew best in the macrophage-like cell line J774, survived at moderate levels in splenic and bone marrow-derived macrophages, and was killed most efficiently in peritoneal macrophages. Macrophage-sensitive mutants transduced into a smooth background were also less virulent than the parent, with a 50% lethal dose of 2 to 5 logs greater than that of the parental strain. These experiments demonstrate that survival of S. typhimurium within macrophages varies with the source of cells, with a distinct ability to survive in macrophages from mouse spleens, where S. typhimurium grows rapidly. These experiments also demonstrate the heterogeneity in intracellular survival among the various macrophage-sensitive mutants, which may reflect the relative importance of the individual mutated genes in survival within macrophages.
Full text
PDF

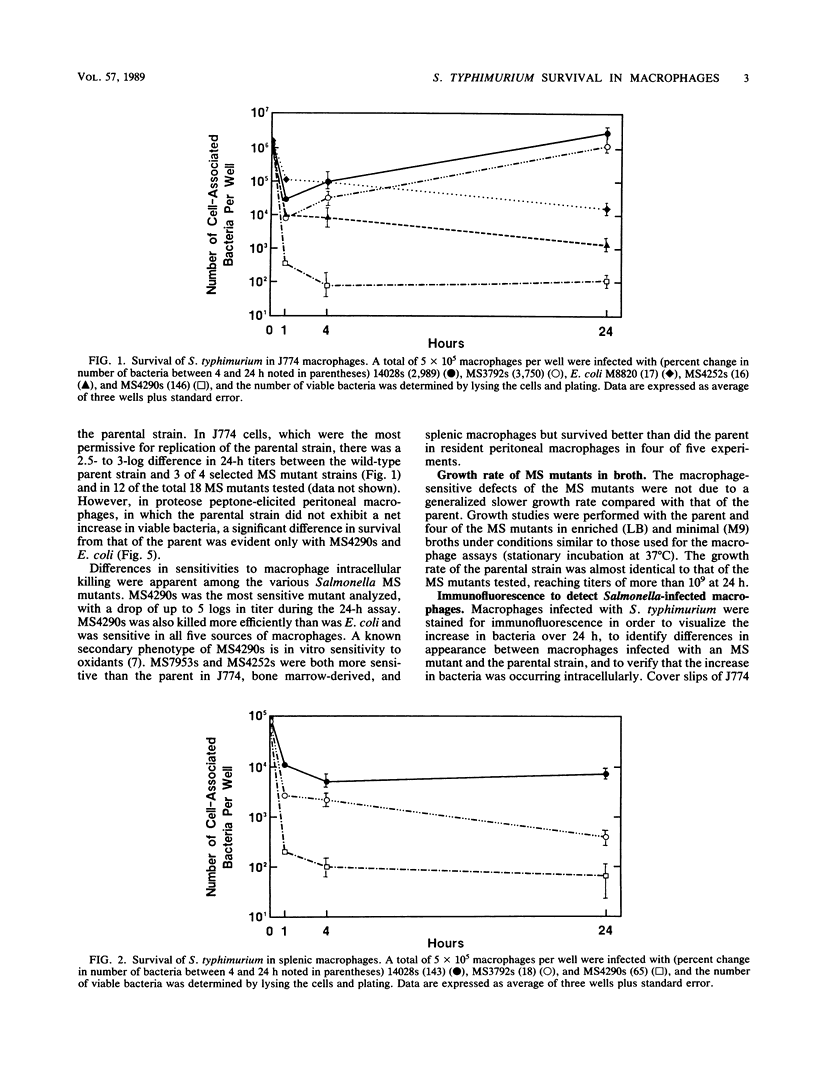
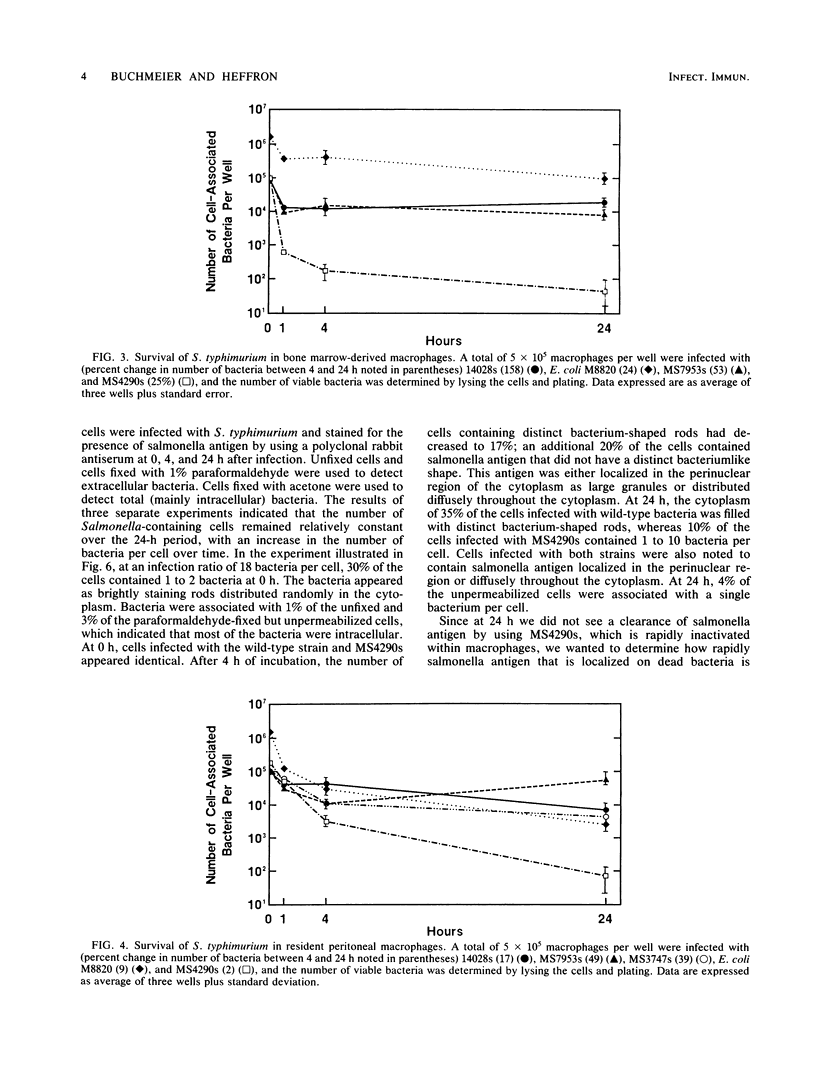
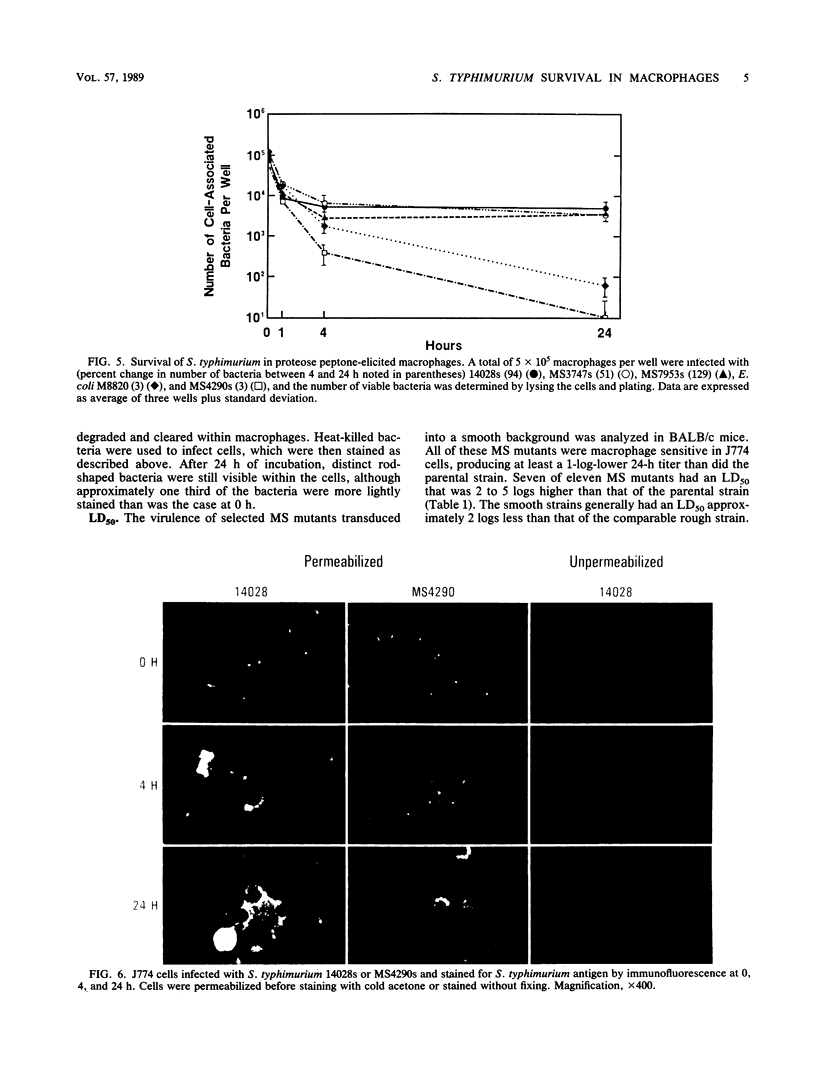


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeda H., Mitsuyama M., Tatsukawa K., Nomoto K., Takeya K. The synergistic contribution of macrophages and antibody to protection against Salmonella typhimurium during the early phase of infection. J Gen Microbiol. 1981 Apr;123(2):209–214. doi: 10.1099/00221287-123-2-209. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cruz E., Ulich T., Schreiber R. D. In vivo activity of lymphokine-activated macrophages in host defense against neoplasia. J Immunol. 1985 May;134(5):3489–3496. [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A. Antimicrobial properties of Kupffer cells. Infect Immun. 1988 Jun;56(6):1430–1435. doi: 10.1128/iai.56.6.1430-1435.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. N., Hsu H. S., Mumaw V. R., Nakoneczna I. Electronmicroscopy studies on the bactericidal action of inflammatory leukocytes in murine salmonellosis. J Med Microbiol. 1986 Mar;21(2):151–159. doi: 10.1099/00222615-21-2-151. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T., Tassin M. T., Ryter A. Bacterial antigen immunolabeling in macrophages after phagocytosis and degradation of Bacillus subtilis. Infect Immun. 1988 Feb;56(2):468–478. doi: 10.1128/iai.56.2.468-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf E. S., O'Brien A. D. Characterization of murine antibody response to Salmonella typhimurium by a class-specific solid-phase radioimmunoassay. Infect Immun. 1981 Jan;31(1):33–41. doi: 10.1128/iai.31.1.33-41.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Schreiber R. D., Altman A., Katz D. H. Identification of a T cell hybridoma that produces large quantities of macrophage-activating factor. J Exp Med. 1982 Sep 1;156(3):677–689. doi: 10.1084/jem.156.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L. Dissociation of bactericidal activity from other functions of activated macrophages in exudates induced by thioglycolate medium. Infect Immun. 1981 Oct;34(1):274–284. doi: 10.1128/iai.34.1.274-284.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., Carsiotis M., Lissner C. R., O'Brien A. D. Flagella help Salmonella typhimurium survive within murine macrophages. Infect Immun. 1984 Dec;46(3):819–825. doi: 10.1128/iai.46.3.819-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]