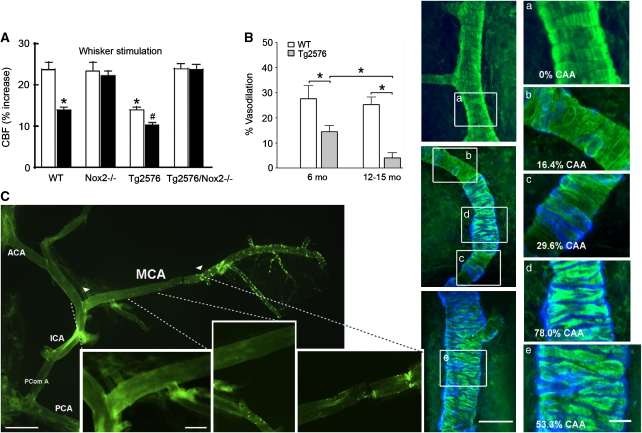
Figure 2.
The role of soluble and insoluble Aβ in cerebrovascular dysfunction of amyloid precursor protein (APP) mice. (A) The cerebral blood flow (CBF) increase to whisker stimulation is impaired in 3- to 4-month-old Tg2576 mice lacking Aβ deposits, and more so in 12-month-old Tg2576 animals, as assessed through open cranial window and laser Doppler flowmetry (LDF). Deficits were absent in Tg2576 mice missing the Nox2 catalytic NADPH oxidase subunit. This implicates soluble Aβ-induced oxidative stress in the dysfunctions. White bars, 4 months old; Black bars, 12 months old; WT, wild type; *, different from WT; #, different from young Tg2576 mice. Reproduced from Park et al (2008) by The National Academy of Sciences of the USA. (B) Hypercapnia-induced dilatation is impaired in 6-month-old Tg2576 mice lacking cerebral amyloid angiopathy (CAA), and further impaired in 12- to 15-month-old animals with increasing CAA severity and smooth muscle cell disarrangement, the latter illustrated and quantified in panels (a–e). CAA: methoxy-X04 stained blue; smooth muscle cells: phalloidin stained green. Scale bars: left, 50 μm; right, 20 μm. Reproduced from Han et al (2008) with permission conveyed through Copyright Clearance Center, Inc. (C) Reduced cerebrovascular responsiveness in the proximal CAA-free segment of the middle cerebral artery (MCA) (delineated by arrowheads, with first, second, and third subsegments magnified in the three panels) suggests effects of soluble Aβ in a 12-month-old J20 APP mouse. Scale bars: left, 0.5 cm; right, 0.2 cm. ACA, anterior cerebral artery; ICA, internal carotid artery; PComA, posterior communicating artery; PCA, posterior cerebral artery. Reprinted from Tong et al (2009) with permission from Elsevier.