Abstract
Stem cells with the potential to form many different cell types are actively studied for their possible use in cell replacement therapies for several diseases. In addition, the differentiated derivatives of stem cells are being used as reagents to test for drugs that slow or correct disease phenotypes found in several degenerative diseases. This paper explores these approaches in the context of type 1 or juvenile diabetes, pointing to recent successes as well as the technical and theoretical challenges that lie ahead in the path to new treatments and cures.
Keywords: pancreas, diabetes, stem cells, juvenile diabetes, type 1 diabetes, chemical screening
1. Introduction
Research scientists and clinicians are exploring the use of stem cells to study and treat degenerative diseases. With respect to the latter, my colleagues and I are focused on juvenile or type 1 diabetes (T1D). T1D is a debilitating disease that affects millions of patients, most often children, requiring them to monitor their blood glucose levels by pricking their finger to collect a drop of blood for sugar measurement on a glucometer. Based on the glucose content of their blood, their anticipated exercise and food intake and their recent blood glucose readings, the patient will inject insulin with the intent of keeping their blood sugar levels in the ‘normal range’ (approx. 80–110 mg dl–1). With regular blood glucose monitoring, insulin injections, and an intense attention to diet and exercise, some T1D patients can lead a nearly normal life. However, for many, the lack of perfect blood glucose control leads to severe complications, including kidney, eye and heart dysfunction, peripheral neuropathies and a premature demise. The aim of our work is to find new treatments for this degenerative disease.
2. An autoimmune attack kills pancreatic β cells
The scientific basis for T1D can be simply described as an autoimmune attack that results in the destruction of pancreatic insulin-producing β cells. Without β cells, the patient is rendered insulin-dependent, requiring the blood sugar monitoring and insulin injections mentioned above. There is much that remains mysterious about the causes of T1D. The cellular components of the disease include the target (β cells), the effectors (cytotoxic immune (T) cells) and the thymus that is evidently defective in adequately monitoring the self/non-self education of, at least, the cytotoxic T cells. What is not yet understood is which cells initiate the disease, e.g. is it defective β cells that attract the attention of the immune system or immune cells that incorrectly attack perfectly normal β cells? What role is played by antigen-presenting cells, e.g. dendritic cells in the pancreatic lymph nodes? And how many different paths to disease are there in the human population, i.e. are there many ways to get diabetes?
It should also be noted that while there is a strong genetic predisposition to the disease [1,2], there are cases of identical twins wherein one twin has the disease and the other does not. This fact points to the existence of as yet unknown environmental stimuli or stochastic events that lead to disease progression.
There are excellent reviews on the pathophysiology, genetics and possible causes of T1D (e.g. [3]). This paper is not intended to summarize all the findings and ideas, nor review the current state of the field. Instead, it outlines one approach to studying T1D. To that end, the challenge can be divided into two parts: (i) understanding the autoimmune attack and (ii) replacing β cells.
(a). Understanding the autoimmune defect in type 1 diabetes
T1D patients have cytotoxic T cells that specifically recognize and eliminate their pancreatic β cells. There is evidence for a number of autoreactive immune cells that recognize different β cell antigens, including insulin, GAD56 and others [4]. These autoreactive T cells are capable of transferring or conferring the disease: T cells isolated from a diabetic non-obese diabetic (NOD) mouse can cause T1D when injected into a recipient [5]. But what is missing from our understanding is the initiating event that causes the disease. For example, what are the first or initiating antigens, those that precede the epitope spreading that confounds antigen analysis in patients that have already lost β cell function and present with high blood sugars?
Much has been learned from studying a mouse model of T1D, the NOD mouse. The NOD mouse and the conclusions that can be drawn from its study have been extensively reviewed [6]. In general, two theoretical concerns can be raised about the relevance of the NOD mouse for human T1D. First, obviously, it will always be difficult to know whether the disease in the NOD mouse is an accurate reflection of what happens in the pathogenesis of human T1D. Secondly, this mouse strain is probably best considered to be the equivalent of one patient, one instance of the disease arising. Thus, it leaves open the question of how many different ways the human T1D develops. These concerns have been discussed before, and as noted by von Herrath & Nepom [7], none of the insights obtained from studies on NOD mice have yet translated effectively in human clinical trials.
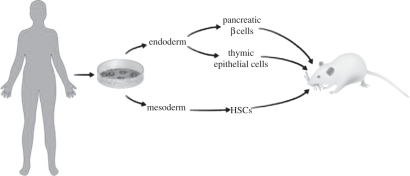
Given these concerns, and the extensive studies that have been published and are ongoing using the NOD mouse, we propose to study the human disease using human cells. Specifically, we propose to reconstruct human T1D in an immunocompromised mouse so that we can watch the disease develop. In this way, it may be possible to determine which cells, and which genetic activates, show the first signs of trouble. To this end, as shown in figure 1, we will prepare induced pluripotent stem cells (iPS) from human diabetic patients to be used as the staring material. These cells will then be differentiated into the components involved in the disease, beginning with pancreatic β cells, thymic epithelium and immune cells.
Figure 1.
Reconstructing human T1D using stem cells. This scheme shows the derivation of induced pluripotent stem cells from skin biopsies obtained from patients with T1D. These pluripotent stem cells (DiPS) will be used to derive three of the major tissue and cell types involved in T1D. These are (i) the blood and immune cells that develop from haematopoietic stem cells (HSC) and form T and B cells, as well as dendritic cells and macrophages, (ii) medullary and cortical thymic epithelial cells that ‘educate’ the immune system, and (iii) the target cell, pancreatic β cells. All of these components will be transplanted into an immunocompromised mouse that accepts human cells and human foetal tissues without rejection. In this case, a NOD, SCID, IL2R-γnull (NSG) mouse serves as the transplantation host.
T1D patients, having been fully informed and consented to donate a skin biopsy, provided the starting material for preparing diabetic iPS cells (DiPS). Following the method first developed by Yamanaka and colleagues [8], we have so far prepared 23 separate DiPS lines from 10 different patients of various ages (ages 21–46). These cell lines may or may not have genetic modifications caused by the viruses used to induce pluripotency, genetic modifications that could affect the interpretation of experiments performed with differentiated derivatives of these stem cells. Thus, going forward, we will use the method developed by Rossi and colleagues [9] to prepare a new cohort of DiPS cells without genetic modification.
The technical challenges for this project are non-trivial. In order to observe human T1D, we will need to direct differentiation of the DiPS cells into pancreatic β cells, thymic epithelia and haematopoietic stem cells (HSCs), in the least. It may also be necessary to arrange for other human components of the disease, including a human endothelia, lymph nodes and more. And all of these cell types will have to be engrafted into immunocompromised mice wherein they can function and interact. In addition, there remain the unknown aspects of disease pathobiology that could complicate this analysis. Specifically, the variability observed in the human population may be difficult to reproduce using DiPS cells and, indeed, the DiPS cells themselves may show variability in their developmental potential.
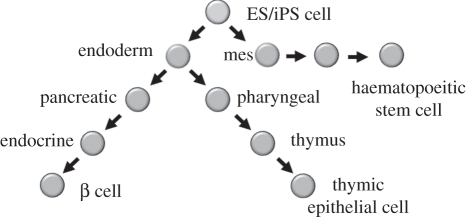
Of the three key cell types, our work is most advanced on the pancreatic β cells (described below). For thymic epithelium, we have adopted the straightforward approach of trying to direct differentiation of stem cells through the stages that mimic normal development. As shown in figure 2, this involves differentiation to definitive endoderm, followed by pharyngeal foregut (marked by Pax9 and Sox2 expression) and then the thymic progenitor (marked by FoxN1) that will go on to form thymic epithelium [11]. To date, we are able to make definitive endoderm with efficiencies of up to 80 per cent of the starting material, embryonic stem (ES) cells, being converted. Thereafter, to the step of Pax9 positive cells, the efficiency drops off dramatically to only a few per cent of the cells becoming Pax9 positive. The challenge going forward will be to increase the efficiency with which we can direct differentiation and to develop functional assays for further differentiation of the progenitors.
Figure 2.
Stepwise differentiation from pluripotent stem cells of the key components for T1D. The directed differentiation of multiple cells types involves a stepwise instruction from a stem cell to progenitors and eventually to the mature cell types of interest. Each of the steps is assessed using specific gene expression patterns that have been determined using transcriptional arrays on dissected or fluorescence activated cell sorted embryonic cells for the relevant cell type as exemplified for the pancreas [10].
The development of functional immune effector cells, including T and B cells, from DiPS will be done by providing humanized mice with human HSCs. This challenging project, done in collaboration with the laboratories of Derrick Rossi and George Daley, has the aim of making functional HSCs from DiPS cells.
Finally, the vessel or living test tube for all these cell types will be an immunocompromised ‘humanized’ mouse. The NSG mouse strain (NOD, SCID, IL2R-γnull), created in the laboratories of Shultz & Greiner [12], is based upon the original NOD-scid mouse. The NSG mouse harbours a targeted mutation in the IL-2 receptor γ-chain, not found in the NOD-scid strain. In comparison with NOD-scid, NSG mice support greatly enhanced engraftment of human tissues, HSCs and peripheral blood mononuclear cells.
The goal of reconstructing human T1D in a humanized mouse has significant challenges before it can be fully realized. We are encouraged by preliminary results obtained by transplanting human foetal pancreatic progenitors, human foetal thymic progenitors and human foetal liver (to make blood and immune cells), all of which have shown significant development of differentiated cell types following transplantation.
3. Replacing pancreatic β cells
Research from our laboratory and others has pointed to at least three ways in which new β cells might be provided to diabetics. Several studies point to the importance of self-duplication or replication as the major mechanism by which pancreatic β cells are replenished in adult mice and humans [13]. Thus, stimulating β cell replication is one way in which β cell mass could be restored in diabetics. However, in T1D, most if not all of the β cell mass has been destroyed and it is unclear whether the residual β cells could be stimulated to replicate to a mass sufficient to control blood sugar levels. This would, of course, require that the immune attack on the newly replicated β cells was stopped or blocked so that the β cells could function. For type 2 diabetics, boosting the β cell mass by stimulating β cell replication is likely to have clinical benefit.
A second approach to replacing β cells is to reprogramme adult differentiated cells to become β cells. For example, adult exocrine cells can be directly reprogrammed into insulin-producing cells by the expression of just three transcription factors. Temporary expression of Pdx1, Ngn3 and MafA, by viral infection of the pancreas in vivo, leads to the conversion of exocrine cells to endocrine cells [14]. Similarly, the glucagon-producing α cells of adult islets can be converted into insulin-producing β cells [15]. The direct conversion of one cell type into another would best be coupled with an amplification step in at least one part of the procedure as the starting material is unlikely to be available with an unlimited supply. If the mechanisms for this type of direct conversion or transdifferentiation into β cells can be elucidated it may be possible to apply this approach to other cell types. In particular, hepatocytes are an especially attractive cell from which to begin as these cells can regenerate and replace those removed for the transdifferentiation to β cells. Overall, it remains to be determined whether β cell replication or transdifferentiation into β cells could be used in vivo in a clinical setting as given our present understanding the approach requires some form of gene therapy.
The third approach to make more β cells is to direct the differentiation of stem cells, either embryonic or pluripotent stem cells, into functional β cells. The overall idea is to recapitulate the normal stepwise progression from embryonic pluripotent cells to fully differentiated β cells, taking cues from normal development to identify the signals and characterize each step along the way. We and others have explored this approach by varying culture conditions, the application of growth factors and the provision of small molecules as signalling inducers. This latter approach, screening for small cell permeable molecules that can direct differentiation, will be considered in more detail here.
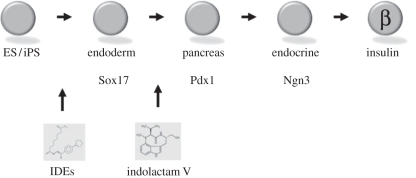
As shown in figure 3, we set up a screening system based on the stepwise differentiation to definitive endoderm, pancreatic precursors, endocrine precursors and finally, mature β cells. The first step, differentiation from an ES cell to definitive endoderm, is assessed by the formation of Sox17+ cells. Using both mouse and human ES cells, in the former case with a fluorescent reporter driven by the Sox17 promoter in mice, we screened for chemicals that can direct differentiation. Two compounds called inducers of definitive endoderm (IDE1 and 2) were identified. These are quite effective in making Sox17 positive cells from both mouse and human ES cells [16]. In the best case, up to 80 per cent of the ES cells form Sox17+ cells, cells that express nearly all of the genes consistent with definitive endoderm [16]. A similar approach was used to find chemicals that can form pancreatic progenitors, marked by Pdx1 expression [17]. In this case, indolactam V induces expression of Pdx1 cells from definitive endoderm.
Figure 3.
Chemical induction of stem cells into cells of the pancreatic lineage. Embryonic or iPS cells can be induced to form cells in the pancreatic lineage by the provision of small cell permeable molecules. Inducers of definitive endoderm [16], abbreviated IDEs, can convert embryonic stem cells to Sox 17+ cells that represent definitive endoderm. Similarly, indolactam V [17] can convert definitive endoderm to pancreatic progenitors that are Pdx1+. The low efficiencies of conversion for each step of the process remains a major challenge.
In both cases, the chemical inductions are stage specific in terms of directing differentiation from one precursor step to the next. In other words, IDEs do not act to induce definitive endoderm cells to become Pdx1+ cells, nor does indolactam V induce ES cells to become Sox17+ cells.
The chemically induced endoderm is very similar to its in vivo counterpart. Data from transcriptional microarrays showed that the chemically induced endoderm is virtually identical to Sox17 positive cells isolated by fluorescent activated cell sorting from mouse embryos. Consistent with this, transplantation experiments were performed by injecting chemically induced endoderm into the developing gut tube of living mouse embryos. Some of the injected cells integrate into the developing gut and go on to express Pdx1. Unfortunately, development of the injected embryos proceeds for only 2–3 days; thus it has so far not been possible to show that the chemically induced derivatives of ES cells can go on to form functional β cells.
There remain at least two important steps in this programme that have yet to be achieved. First, finding small molecules that efficiently induce the endocrine progenitors, Ngn3+ cells. Following that, these endocrine progenitors must be induced to form glucose-responsive, insulin-secreting β cells. The transplantation of Pdx1 cells into mice shows that insulin-secreting cells can be produced from these ES cell derivatives [18], but the exact lineage of the subsequent cells remains unclear and it has not been possible to accurately measure the efficiency of the process. Thus, while the principal that ES cells can give rise to insulin-secreting cells has been demonstrated, we await well-defined and reproducible conditions that can achieve this goal at a scale that is compatible with significant analysis for research let alone transplantation into humans.
4. Summary
The challenges of understanding the causes of T1D are significant. We propose a way forward using human stem cells in a project aimed at understanding the initial or primary cause of the disease. If it is possible to reconstruct the human disease in an immunocompromised mouse, then one should be able to determine which cells act to initiate the disease. And this type of mechanistic understanding opens the door to treatment and prevention. A separate and related goal is to make more β cells from pluripotent stem cells. Progress in this area suggests that it should be possible to make glucose-responsive, insulin-secreting cells. At this point, one is encouraged by the fact that endocrine precursors can be derived from ES cells and it appears that the final steps to mature β cell formation are in sight.
References
- 1.Todd J. A., et al. 2007. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 39, 857–864 10.1038/ng2068 (doi:10.1038/ng2068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedicte A. L., et al. 1999. The predisposition to type 1 diabetes linked to the human leukocyte antigen complex includes at least one non-class II gene. Am. J. Hum. Genet. 64, 793–800 10.1086/302283 (doi:10.1086/302283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Belle T. L., Coppieters K. T., Von Herrath M. G. 2011. type 1 diabetes: etiology, immunology and therapeutic strategies. Physiol. Rev. 91, 79–118 10.1152/physrev.00003.2010 (doi:10.1152/physrev.00003.2010) [DOI] [PubMed] [Google Scholar]
- 4.Pihoker C., Gilliam L. K., Hampe C. S., Lernmark A. 2005. Autoantibodies in diabetes. Diabetes 54(Suppl. 2), S52–S561 10.2337/diabetes.54.suppl_2.S52 (doi:10.2337/diabetes.54.suppl_2.S52) [DOI] [PubMed] [Google Scholar]
- 5.Rohane P. W., Shimada A., Kim D. T., Edwards C. T., Charlton B., Shultz L. D., Fathman C. G. 1995. Islet infiltrating lympocytes from prediabetic NOD mice rapidly transfer diabetes to NOD-scid/scid mice. Diabetes 44, 550–554 10.2337/diabetes.44.5.550 (doi:10.2337/diabetes.44.5.550) [DOI] [PubMed] [Google Scholar]
- 6.Chaparro R. J., DiLorenzo T. P. 2010. An update on the use of NOD mice to study autoimmune (type 1) diabetes. Expert Rev. Clin. Immunol. 6, 939–955 10.1586/eci.10.68 (doi:10.1586/eci.10.68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Herrath M., Nepom G. 2009. Animal models of human type 1 diabetes. Nat. Immunol. 10, 129–132 10.1038/ni0209-129 (doi:10.1038/ni0209-129) [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 10.1016/j.cell.2007.11.019 (doi:10.1016/j.cell.2007.11.019) [DOI] [PubMed] [Google Scholar]
- 9.Warren L., et al. 2010. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 10.1016/j.stem.2010.08.012 (doi:10.1016/j.stem.2010.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherwood R. I., Chen T. Y., Melton D. A. 2009. Transcriptional dynamics of endodermal organ formation. Dev. Dyn. 238, 29–42 10.1002/dvdy.21810 (doi:10.1002/dvdy.21810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleul C. C., Corbeaux T., Reuter A., Fisch P., Mönting J. S., Boehm T. 2006. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441, 992–996 10.1038/nature04850 (doi:10.1038/nature04850) [DOI] [PubMed] [Google Scholar]
- 12.Brehm M. A., Shultz L. D., Greiner D. L. 2010. Humanized mouse models to study human diseases. Curr. Opin. Endocrinol. Diabetes Obes. 17, 120–125 10.1097/MED.0b013e328337282f (doi:10.1097/MED.0b013e328337282f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dor Y., Brown J., Martinez O. I., Melton D. A. 2004. Adult pancreatic β cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 10.1038/nature02520 (doi:10.1038/nature02520) [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D. A. 2008. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 10.1038/nature07314 (doi:10.1038/nature07314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorel F., Népote V., Avril I., Kohno K., Desgraz R., Chera S., Herrera P. L. 2010. Conversion of adult pancreatic α cells to β cells after extreme β cell loss. Nature 464, 1149–1154 10.1038/nature08894 (doi:10.1038/nature08894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borowiak M., Maehr R., Chen S., Chen A. E., Tang W., Fox J. L., Schreiber S. L., Melton D. A. 2009. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4, 348–358 10.1016/j.stem.2009.01.014 (doi:10.1016/j.stem.2009.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S., et al. 2009. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 5, 258–265 10.1038/nchembio.154 (doi:10.1038/nchembio.154) [DOI] [PubMed] [Google Scholar]
- 18.Kroon E., et al. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 10.1038/nbt1393 (doi:10.1038/nbt1393) [DOI] [PubMed] [Google Scholar]