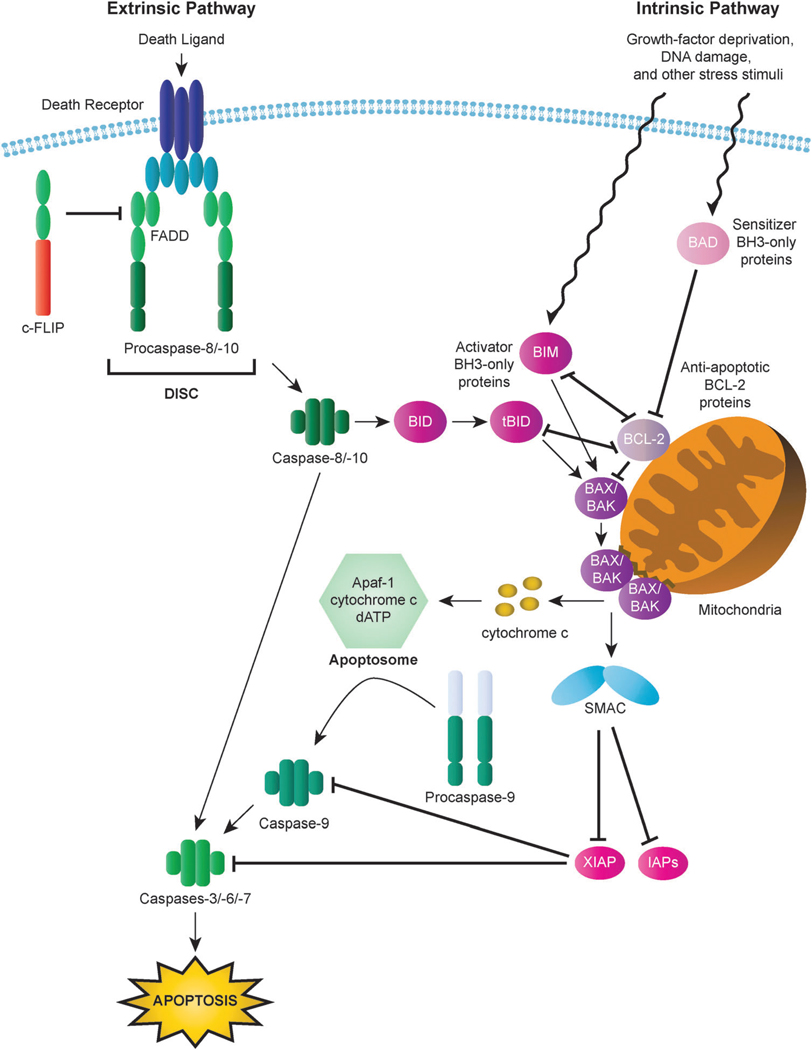
Fig. 1.
Intrinsic and extrinsic apoptotic pathways. In the intrinsic (mitochondrial) pathway, mitochondrial outer membrane permeabilization (MOMP) results in the release of cytochrome c and other apoptogenic factors from mitochondria into the cytosol and the ensuing formation of the apoptosome, which triggers activation of the apoptosis-inducing caspase cascade via activation of caspase-9. Interactions among BCL-2 family proteins play a critical role in the mediating MOMP induction and consequent apoptosis. BH3-only proteins (activator BH3-only proteins are represented here by BIM and tBID and sensitizer BH3-only proteins are represented by BAD) can relay apoptotic signals to the mitochondria through activation of BAX or BAK, the principal effectors of the intrinsic apoptotic pathway. In contrast, anti-apoptotic BCL-2 proteins (represented by BCL-2) serve to inhibit apoptosis by blocking BAX/BAK activation. In the extrinsic (death receptor) pathway, binding of death receptors such as FAS or TRAIL receptors (TRAILR1, TRAILR2) by their cognate ligands triggers the recruitment of death domain (DD)-containing adaptor proteins (represented by FADD) and procaspases with a death effector domain (DED), specifically procaspase-8 and procaspase-10. The resulting complex is known as the death inducing signaling complex (DISC). High levels of active caspase-8 generated by large amounts of procaspase-8 processing at the DISC lead to the activation of executioner caspases, including caspase-3, and the induction of apoptosis. Activation of caspase-8 can also result in the cleavage of the BH3-only protein BID to generate the activated BID fragment tBID, which serves to transmit the death signal from the extrinsic to the intrinsic signaling pathway.