Table 3.
Cu- and Pd- catalyzed arylation of 4-aminophenolsa
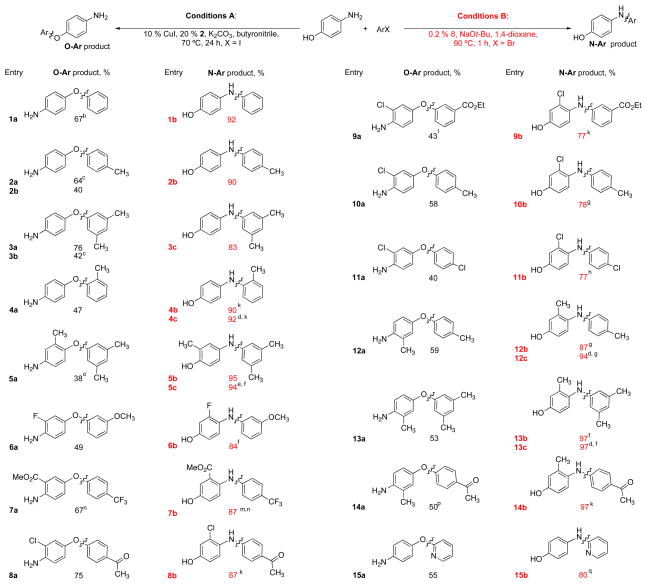
|
Isolated yield, average of two runs.
2% of the N-arylated product was observed.
ArBr.
ArCl.
80 min.
3 h.
24 h.
K2CO3, t-BuOH.
1 % 8.
1 % 8, 24 h.
1% 8, K2CO3, t-BuOH, 24 h.
48 h.
ArCl, K2CO3, t-BuOH, 3 h.
0.5 mmol scale.
16 % of the N-arylated product was observed.
3% of the N-arylated product was observed.
1 % 7, 1% 8, K2CO3, t-BuOH, 110 °C, 24 h.
