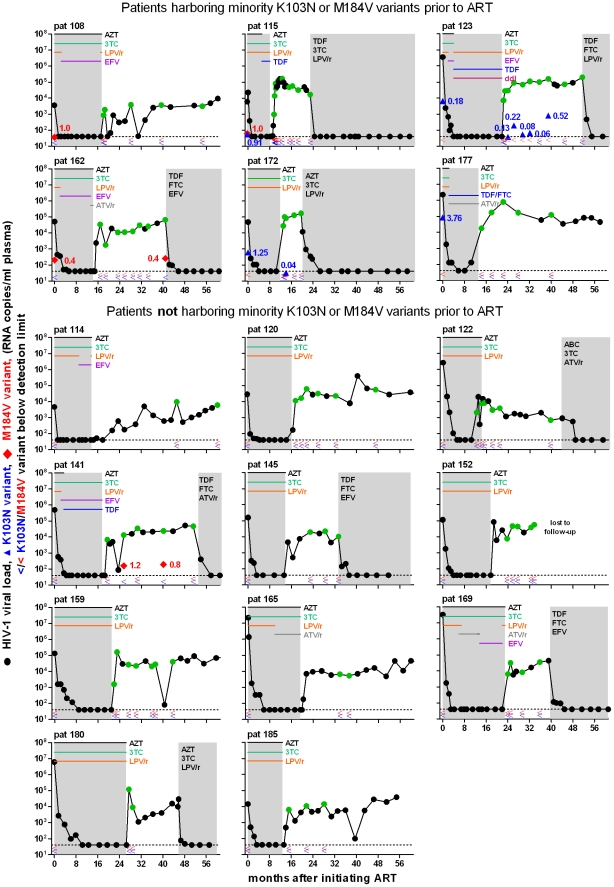
Figure 1. Kinetics of minority K103N and M184V HIV-1 variants in patients receiving early ART and undergoing intended treatment interruption.
Plasma viral load was measured using the Cobas AmpliPrep/Cobas TaqMan HIV-1 Test (black and green circles, the latter represent the time points that were used for the quantification of minority drug-resistant HIV-1 variants). The limit of 40 HIV-1 RNA copies/ml plasma is shown in dashed lines. Periods of ART are depicted in grey shaded areas; the durations of specific drugs in the early ART regimens are indicated by differently coloured lines. In addition, the initial ART regimen after reintroduction of ART is depicted. Minority K103N (blue triangles) and M184V (red diamonds) HIV-1 variants were quantified by AS-PCR; percentages are given and were used to calculate the absolute amounts of these variants based on the viral load. Percent values below the assays discriminatory abilities (0.2% for M184V and 0.01% for K103N) or the individually calculated detection limits based on the viral load are shown by less-than signs. ART, antiretroviral therapy; AZT, zidovudine; 3TC, lamivudine; LPV, lopinavir; /r, ritonavir-boosted; EFV, efavirenz; TDF, tenofovir; ddI, didanosine; ATV, atazanavir; FTC, emtricitabine.