Table 3.
Catalytic reductive coupling reactions directed by a remote alkenea
| enyne | aldehyde | product | yield | |
|---|---|---|---|---|
| 1 |

|
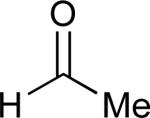
|
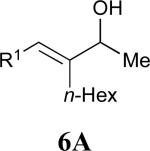
|
69% |
| 2 | 4 |
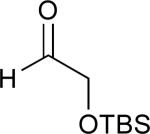
|
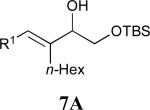
|
58% |
| 3 | 4 |
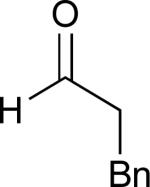
|
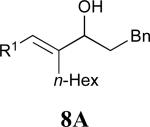
|
60% |
| 4 |
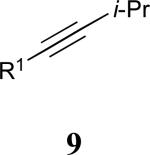
|
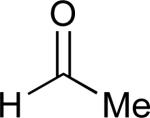
|
|
64% |
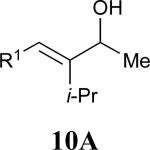
| 5 |
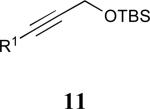
|
|
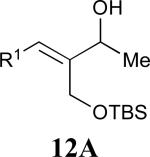
|
62% |
| 6 |
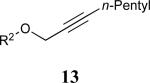
|
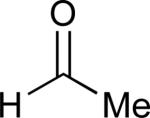
|
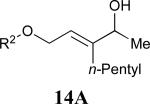
|
60% |
| 7 |
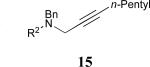
|
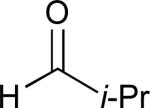
|
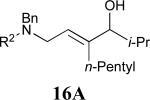
|
62% |
| 8 |
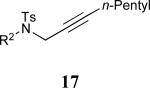
|
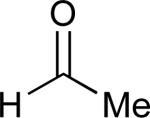
|
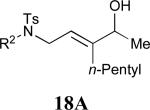
|
68% |
See eq 9, Table 2 for representative reaction. R1 = (CH2)3CH=CH2. R2 = CH2CH=CH2. Regioselectivity > 95:5 in all cases, determined by 1H NMR and/or GC.
