Abstract
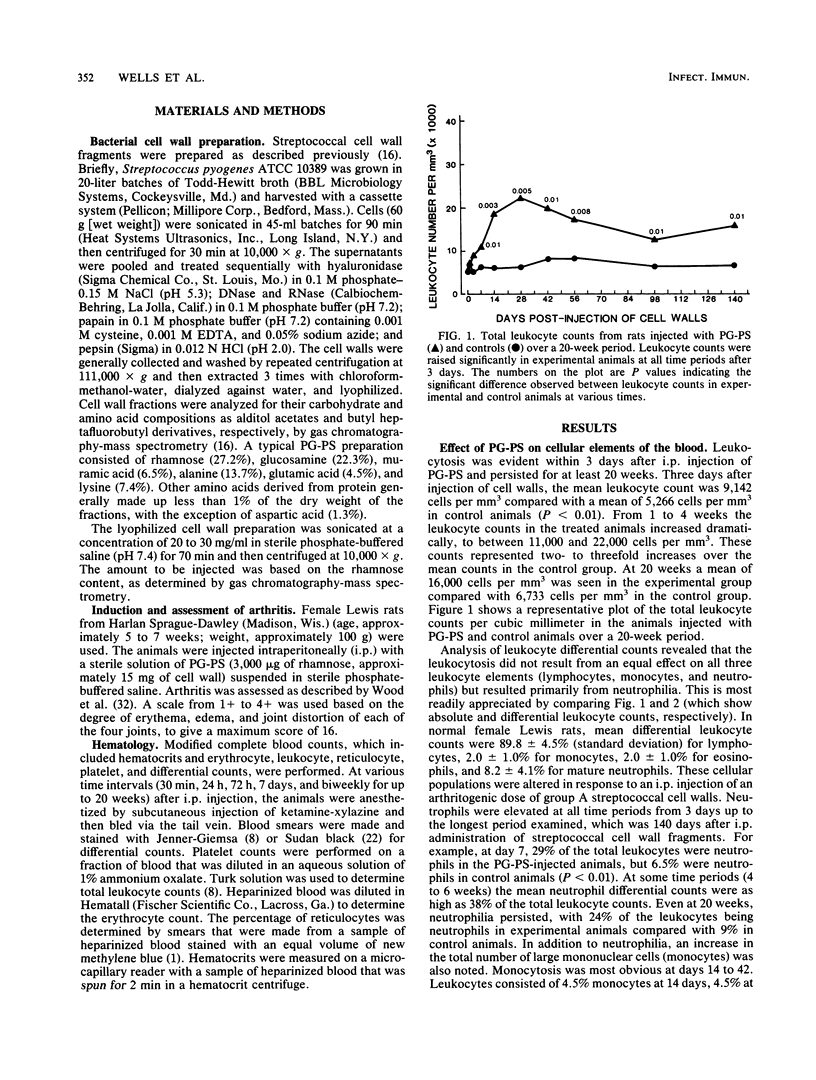
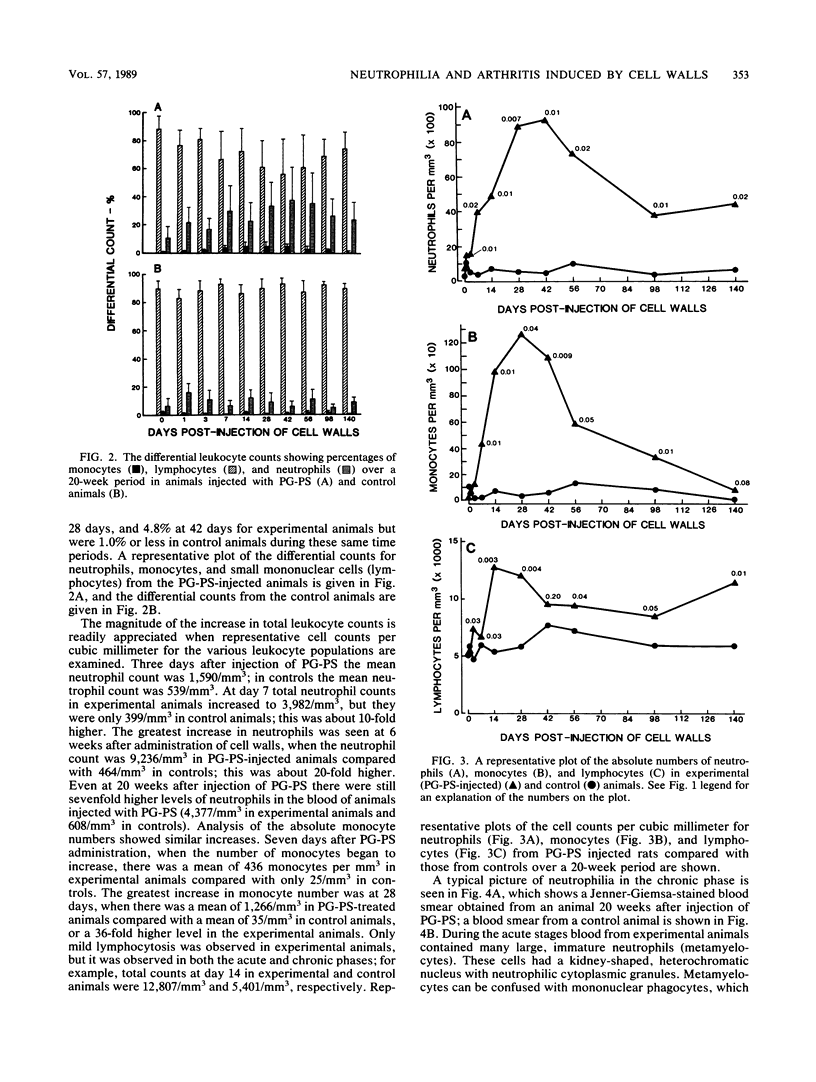
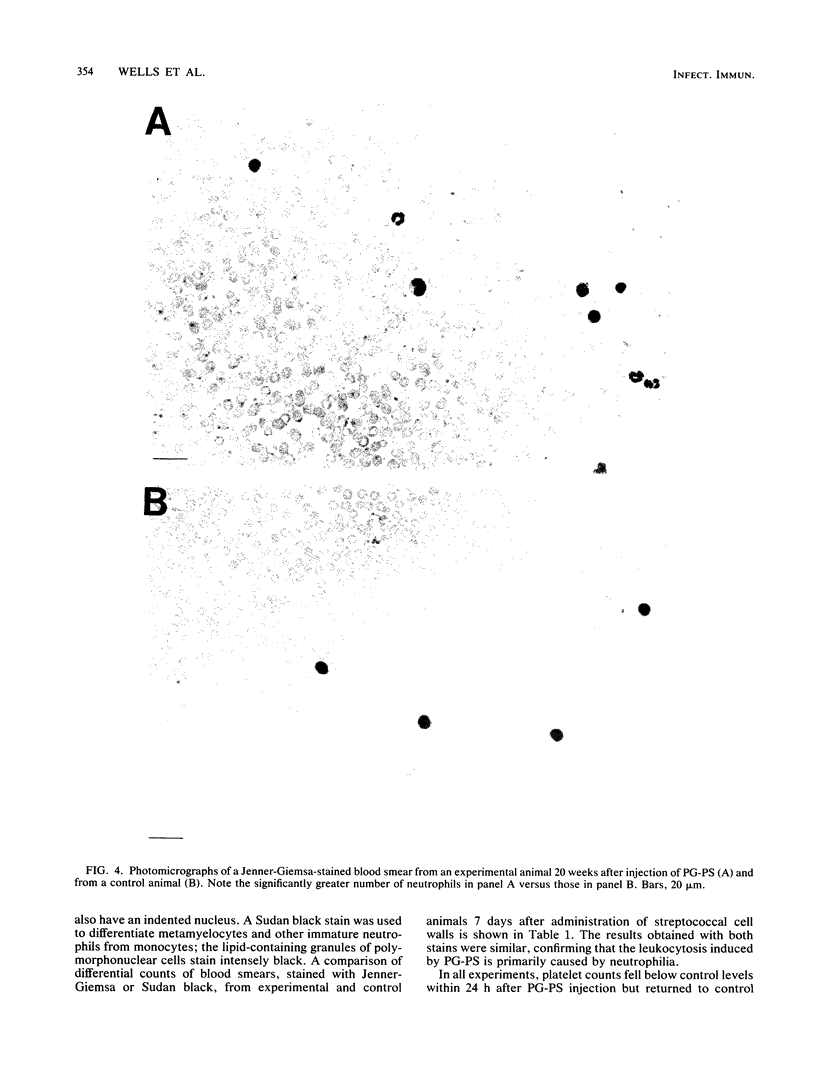
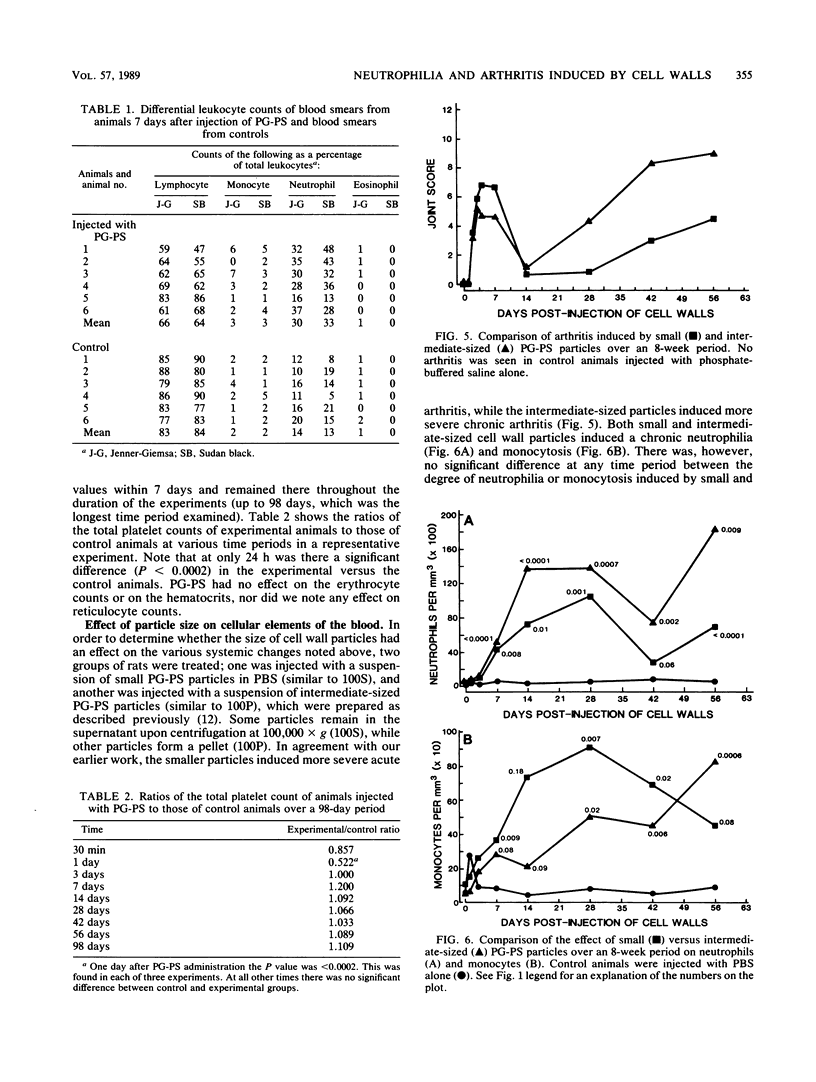
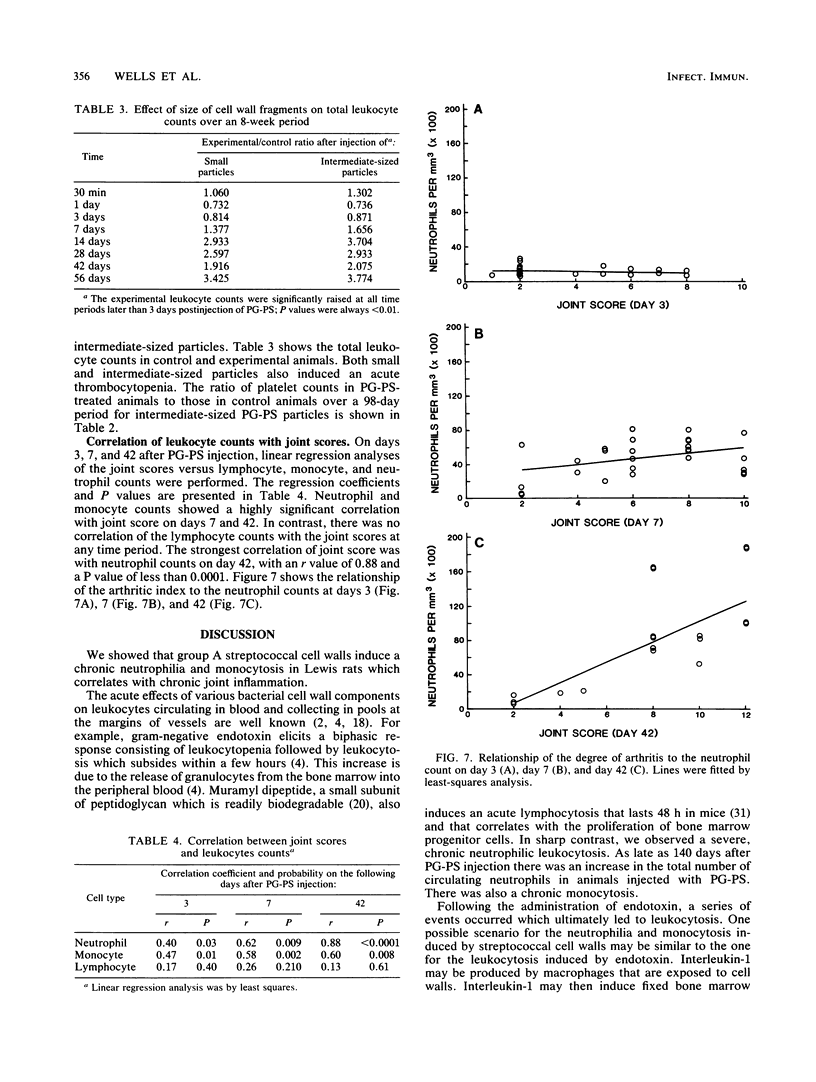
The perpetuation of inflammatory changes within joints elicited by persisting, poorly biodegradable group A streptococcal cell walls (peptidoglycan-polysaccharide complexes [PG-PS]) is well documented. Chronic changes in the bloodstream induced by PG-PS have not been described previously. We demonstrated that leukocytosis occurs within 3 days after intraperitoneal injection of PG-PS and remains elevated 20 weeks later. Chronic neutrophilia, monocytosis, and lymphocytosis were observed in all experiments. Chronic changes in platelet, erythrocyte, and reticulocyte counts were not seen. The newly documented leukocytosis, lasting for months after PG-PS administration, provided a circulating pool of leukocytes that may participate in chronic inflammatory events in the joint. Although the central role of the macrophage in PG-PS-mediated inflammation has been emphasized (F. G. Dalldorf, W. J. Cromartie, S. K. Anderle, R. L. Clark, and J. H. Schwab, Am. J. Pathol. 100:383-402, 1980), the polymorphonuclear cell may be involved in periods of exacerbation of streptococcal cell wall-mediated polyarthritis. This was supported by our observations that neutrophilia and monocytosis correlate well with the degree of chronic joint inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRADDOCK C. G., Jr, PERRY S., VENTZKE L. E., LAWRENCE J. S. Evaluation of marrow granulocytic reserves in normal and disease states. Blood. 1960 Jun;15:840–855. [PubMed] [Google Scholar]
- Chetty C., Klapper D. G., Schwab J. H. Soluble peptidoglycan-polysaccharide fragments of the bacterial cell wall induce acute inflammation. Infect Immun. 1982 Dec;38(3):1010–1019. doi: 10.1128/iai.38.3.1010-1019.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., March L. M., Nelson D. S., Brooks P. M. Interleukin-1 secretion by peripheral blood monocytes and synovial macrophages from patients with rheumatoid arthritis. J Rheumatol. 1987 Feb;14(1):33–39. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. A., Schwab J. H. Arthropathic group A streptococcal cell walls require specific antibody for activation of human complement by both the classical and alternative pathways. Infect Immun. 1986 Aug;53(2):324–330. doi: 10.1128/iai.53.2.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Schwab J. H., Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980 Aug;29(2):526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Fox A. Elimination of group A streptococcal cell walls from mammalian tissues. Infect Immun. 1987 Jun;55(6):1526–1528. doi: 10.1128/iai.55.6.1526-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbart J., Harrison J., Parks C., Fox A. Analysis of the amino acid and sugar composition of streptococcal cell walls by gas chromatography-mass spectrometry. J Chromatogr. 1988 Jun 10;441(2):323–333. doi: 10.1016/s0021-9673(01)83875-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. J., Hunter N., Schwab J. H. Antibody response to streptococcal cell wall antigens associated with experimental arthritis in rats. Clin Exp Immunol. 1980 Dec;42(3):450–457. [PMC free article] [PubMed] [Google Scholar]
- Harada K., Kotani S., Takada H., Tsujimoto M., Hirachi Y., Kusumoto S., Shiba T., Kawata S., Yokogawa K., Nishimura H. Liberation of serotonin from rabbit blood platelets by bacterial cell walls and related compounds. Infect Immun. 1982 Sep;37(3):1181–1190. doi: 10.1128/iai.37.3.1181-1190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J., Fox A. Degradation of muramyl dipeptide by mammalian serum. Infect Immun. 1985 Oct;50(1):320–321. doi: 10.1128/iai.50.1.320-321.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N., Anderle S. K., Brown R. R., Dalldorf F. G., Clark R. L., Cromartie W. J., Schwab J. H. Cell-mediated immune response during experimental arthritis induced in rats with streptococcal cell walls. Clin Exp Immunol. 1980 Dec;42(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Ridge S. C., Zabriske J. B., Oronsky A. L., Kerwar S. S. Streptococcal cell wall arthritis: studies with nude (athymic) inbred Lewis rats. Cell Immunol. 1985 Nov;96(1):231–234. doi: 10.1016/0008-8749(85)90354-5. [DOI] [PubMed] [Google Scholar]
- Rýc M., Rotta J. Biological characteristics of peptidoglycans of group A streptococcus and some other bacterial species. II. Immunological mechanisms involved in thrombocytolysis. J Hyg Epidemiol Microbiol Immunol. 1978;22(4):435–441. [PubMed] [Google Scholar]
- Schwab J. H., Allen J. B., Anderle S. K., Dalldorf F., Eisenberg R., Cromartie W. J. Relationship of complement to experimental arthritis induced in rats with streptococcal cell walls. Immunology. 1982 May;46(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- Wuest B., Wachsmuth E. D. Stimulatory effect of N-acetyl Muramyl dipeptide in vivo: proliferation of bone marrow progenitor cells in mice. Infect Immun. 1982 Aug;37(2):452–462. doi: 10.1128/iai.37.2.452-462.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum D. E., Allen J. B., Wahl S. M., Calandra G. B., Wilder R. L. Inhibition by cyclosporin A of streptococcal cell wall-induced arthritis and hepatic granulomas in rats. Arthritis Rheum. 1986 Feb;29(2):262–273. doi: 10.1002/art.1780290215. [DOI] [PubMed] [Google Scholar]



