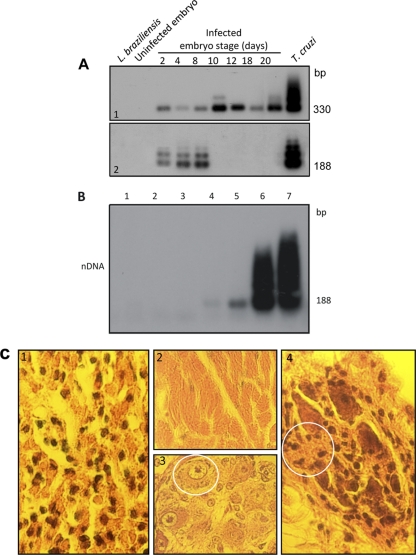
Fig. 13.
Evidence of kDNA integration into germ line cells and tissues from birds hatched from T. cruzi-infected eggs, with accompanying pathology. (A) Establishment of T. cruzi infection early in embryonic development followed by elimination early in Gallus gallus embryonic development. (Top) Bands (330 bp) formed by PCR-amplified minicircle kDNA templates harvested at several stages of chicken embryonic development, after hybridization with a specific probe. (Bottom) Bands formed by PCR amplified from the same embryos after separation on a 1% agarose gel and hybridization with a specific nDNA probe. The 188-bp nDNA band is diagnostic of the parasite persistence in the host tissue. (B) Sensitivity of PCR with nDNA primers Tcz1 and Tcz2. Lanes 1 and 2, control DNA from kDNA-negative and from kDNA-mutated chickens; lanes 3 to 7, mix of 200 ng of control chicken DNA with increasing amounts of T. cruzi DNA, 1 fg, 10 fg, 1 pg, 100 pg, and 1 ng, respectively. Hybridization with the radiolabeled 188-bp probe improved the sensitivity of the technique (10 fg), which is 24-fold below that for single T. cruzi total DNA. (C) Destructive myocarditis and ganglionitis in a 2-week-old F1 chick. (1) Histopathological section showing mononuclear cell infiltration and lysis of target heart cells. (2) Normal histological features of the myocardium. (3) Intracardiac ganglion cells from a control offspring of a noninfected chick. (4) Section of an intracardiac parasympathetic ganglion showing lymphocytic infiltration and dropout (circle) of neuronal cells. (Modified from reference 293 with permission of the publisher [see comments regarding this retracted article at first citation] and from PLoS Neglected Tropical Diseases [402].)