Table 1.
syn-Diastereo- and enantioselective carbonyl crotylation from the alcohol oxidation level.a
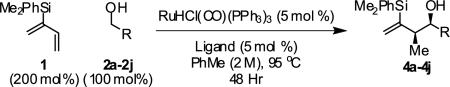 | |||||
|---|---|---|---|---|---|
| Entry | Product | Ligand | Yield [%] | ee [%] | syn:anti |
| 1 |
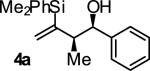
|
A | 81 | 87 | 13:1 |
| 2 |
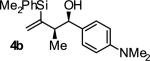
|
A | 87 | 90 | ≥20:1 |
| 3 |
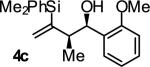
|
A | 84 | 90 | ≥20:1 |
| 4 |
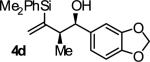
|
B | 85 | 90 | ≥20:1 |
| 5 |
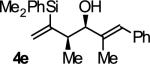
|
B | 70b | 90 | 11:1 |
| 6 |
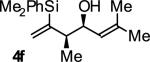
|
A | 67b,c | 90 | ≥20:1 |
| 7 |
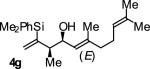
|
A | 70b | 92 | ≥20:1 |
| 8 |
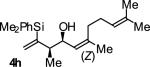
|
A | 64b,c | 86 | ≥20:1 |
| 9 |
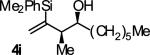
|
A | 65b,c,d | 88 | ≥20:1 |
| 10 |
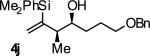
|
A | 66 | 86 | ≥20:1 |
Ligand A = (R)-DM-SEGPHOS, Ligand B = (R)- SEGPHOS. Yields are of isolated material. Diastereoselectivity was determined through 1H NMR analysis of crude reaction mixtures. Enantiomeric excess was determined by chiral stationary phase HPLC analysis. See Supporting Information for details.
250 mol% 1.
THF
7 mol% catalyst.
