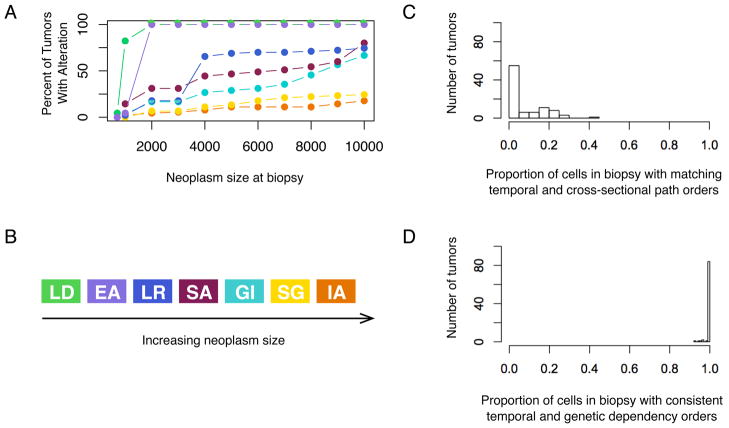
Fig. 5.
Details of a single simulation as it progresses to cancer. (A) Plot of the percent of cells within this neoplasm that contain a given mutation over time. Note that the IA reaches detection early in progression and regresses. (B) Plot of the Shannon index for diversity, or information entropy, over time for the simulation. (C) The top panel shows the clones, their mutational states, and their rough population sizes over time. The height is proportional to the population size of the neoplasm, and new mutations are indicated with an arrow. The bottom panel shows the type of neoplasm that would be identified at various points during progression from normal tissue to cancer, beginning with polyps and ending with cancer. (D) The genealogy, or cell lineage, for all of the clones that arose during the evolution of the neoplasm shows that a single evolutionary run doesn’t have a single evolutionary path. The temporal order of phenotypes is given at the tips of the genealogy. Because we are modeling phenotypes, the same set of phenotypic mutations can occur in clones that are unique by descent. Each new mutation for a phenotype is a new mutation in a gene or pathway conferring the phenotype. Thus, we have what looks like convergent evolution - there is phenotypic homogeneity, but it arose through different genetic alterations. Under these parameters, independent acquisition of hallmarks in different clones is common and leads to clonal interference and the suppression of clonal expansion for any one clone. Note that the most commonly-observed phenotypic order does not correspond to the cross-sectional path order given in Fig. 3B. (E) The genealogy, or cell lineage, for all of the clones that arose during the evolution of the neoplasm pictured in Fig. 2. Both neoplasms pictured here have relatively high genetic heterogeneity at cancer detection. As occurs here, genetic heterogeneity may lead to phenotypic homogeneity. Each mutation is represented by a different color as given in Fig. 3B.