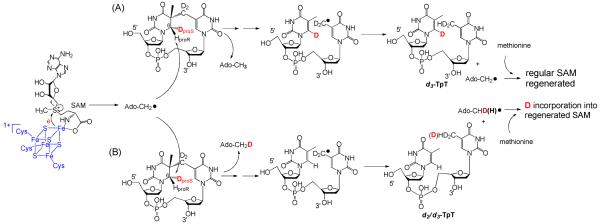
Figure 4.
Using d3-SP TpT (2) as the enzyme substrate to illustrate the expected reaction products according to the current SPL mechanism shown in Scheme 3. (A): The 5′-dA radical abstracts the protium at the 6-HproR position to initiate the reaction. All three deuterium atoms in compound 2 should be retained in the repair product TpT; thus d3-TpT as well as regular 5′-dA and SAM are expected after the reaction. (B): The 5′-dA radical abstracts the deuterium at the 6-HproS position to initiate the reaction. Correspondingly, a deuterium is incorporated into the resulting 5′-dA and d2/d3-TpT and d1/d0-SAM mixtures are expected by the end of the reaction due to the H or D abstraction from the methyl group of 5′-dA by the TpT radical in the H atom back donation step.