Fig. 2.
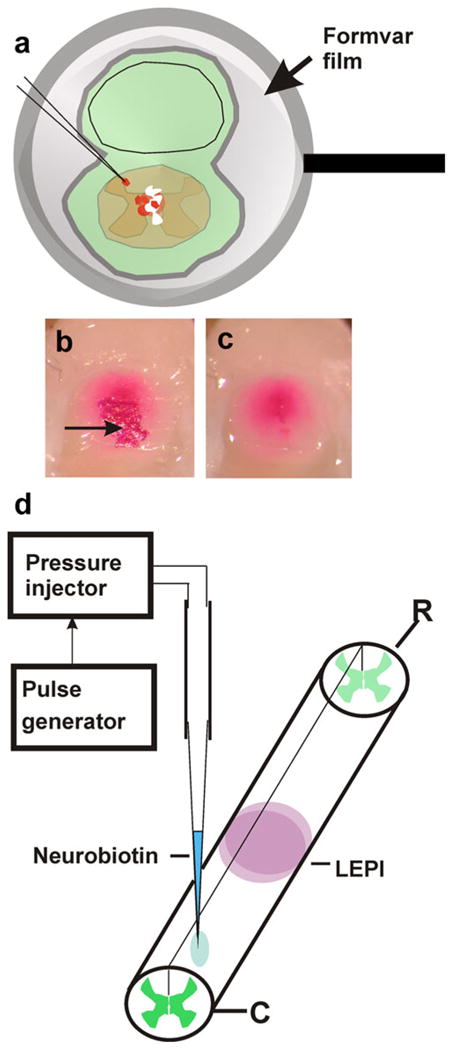
Retrograde neuronal labeling. a To apply 1,1′, di-octadecyl 1–3,3,3′,3′-tetramethylcarbocyanine perchlorate (DiI) crystals to the cut surface of the cord but to reduce the risk of spurious labeling of neighboring tissues, fixed cords and surrounding tissues were covered with an adhering ultrathin Formvar film supported by Sjöstrand-type metal rings. Small openings were made in the films, and DiI crystals were applied to the film-coated cords. Direct contact between the lipophilic dye and tissues was thus reduced to the area underlying the opening in the film. To avoid dispersion of DiI crystals during incubation, each preparation was coated with a second layer of undamaged film. For embedding and sectioning, dye crystals lying outside the central cord region were removed together with the film as shown in b (before film removal) and c (after film removal). The arrow in b indicates a group of DiI crystals. d Neurobiotin injections were performed by pressure-injection with a glass micropipette. The animals were killed 4 days after injection, and the spinal cord was processed as described in Materials and methods (LEPI lesion epicenter, R rostral, C caudal)
