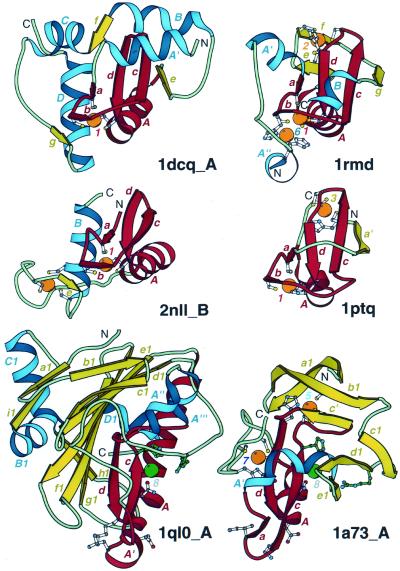
Figure 5.
Treble clef fingers inside larger structures. Structural diagrams of ARF-GAP domain of Pyk2-associated protein β (1dcq, chain A, residues 248–365), RING finger of RAG1 (dimerization domain) (1rmd, residues 1–87), retinoid X receptor α DNA-binding domain (2nll, chain B, residues 300–369), Cys2 activator-binding domain of protein kinase Cδ (1ptq, residues 243–280), S.marcescens endonuclease (1ql0, chain A, residues 6–245) and intron-encoded homing endonuclease I-PpoI (1a73, chain A, residues 21–139). The treble clef finger motif is outlined in red. β-Strands and α-helices that are not part of the motif are shown in yellow and blue, respectively. Side-chains of zinc ligands, residues in sites corresponding to ligands of zinc #1, and active site residues in 1a73 are shown in ball-and-stick representation. Active site residues are colored in green. Zn2+ and Mg2+ are displayed as orange and green balls, respectively. In each protein, N- and C-termini are labeled with N and C. β-Strands and α-helices are labeled in lower and upper case letters, respectively. The color of the letter corresponds to the color of the element. Zinc ions are labeled with numbers corresponding to those in Figure 3.