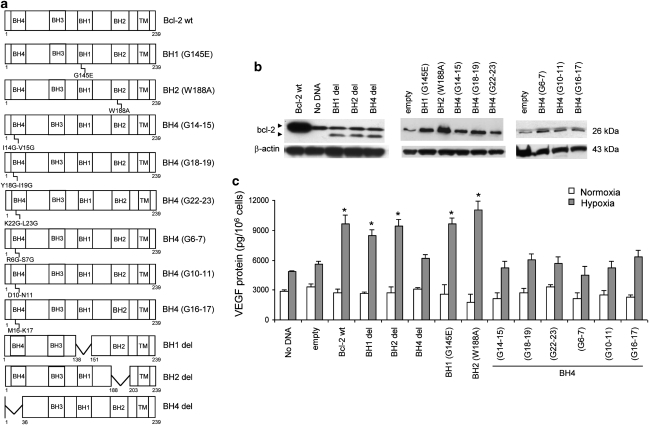
Figure 1.
The BH4 domain, but not BH1 or BH2, is necessary for VEGF induction by wild-type (wt) bcl-2 under hypoxia. (a) A schematic representation and names of structural constructs encoding the human wt bcl-2 or different bcl-2 mutants that have been used throughout the paper: the bcl-2 point mutants at the BH1 domain (G145E, amino acid residue 145 from glycine to glutamic acid), at the BH2 domain (W188A, amino acid residue 188 from tryptophan to alanine), and six different dicodon bcl-2 mutants at the BH4 domain (G14–15, amino acid residues 14 (isoleucine) and 15 (valine) replaced by glycines; G18–19, amino acid residues 18 (tyrosine) and 19 (isoleucine) replaced by glycines; G22–23, amino acid residues 22 (lysine) and 23 (leucine) replaced by glycines; G6–7, amino acid residues 6 (arginine) and 7 (serine) replaced by glycines; G10–11, amino acid residues 10 (aspartic acid) and 11 (asparagine) replaced by glycines; G16–17, amino acid residues 16 (methionine) and 17 (lysine) replaced by glycines); the bcl-2 deleted mutants of BH4 (residues from 1 to 36), BH1 (residues from 138 to 151) or BH2 (residues from 188 to 203) regions. All the mutations of bcl-2 protein, excluding G6–7, G10–11 and G16–17, impair its antiapoptotic activity. (b) Western blot analysis of bcl-2 protein expression on M14 cells untransfected (no DNA) or transiently transfected with empty vector (empty) or with vectors encoding wt or mutated bcl-2 protein. Two bands, indicative of the higher endogenous full-length and shorter exogenous bcl-2 protein, were observed in cells overexpressing bcl-2 deleted of BH1, BH2 or BH4 regions, while a single high band was observed in control cells (untransfected or transfected with empty vector) and in cells overexpressing wt or point-mutated bcl-2 protein. Western blots representative of three independent experiments with similar results are shown. β-actin is shown as loading and transferring control. (c) VEGF protein expression evaluated by ELISA on M14 cells transiently transfected with vectors encoding wt or mutated bcl-2 exposed to normoxia or hypoxia. Results represent the average±S.D. of three independent experiments. P-values were calculated between control and wt or mutated bcl-2-overexpressing cells (*P<0.01)