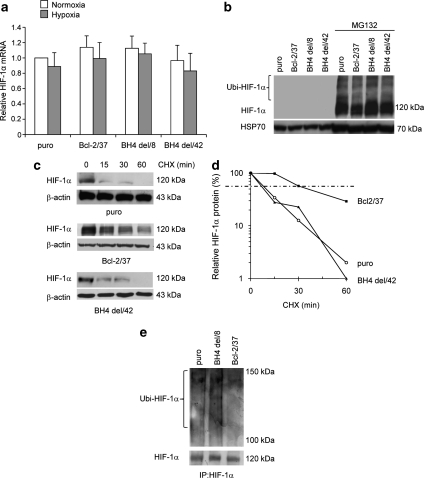
Figure 5.
The BH4 domain is necessary for HIF-1α protein stabilization by wild-type (wt) bcl-2. (a) RT-PCR evaluation of HIF-1α mRNA level, (b and c) western blot analyses of HIF-1α protein expression after the exposure to MG132 proteosomal inhibitor (10 μM) for 6 h in normoxic conditions (b), or to hypoxia for 24 h followed by CHX treatment (50 μg/ml) for the indicated times (c) of M14 melanoma control (puro), a clone-overexpressing wt bcl-2 (Bcl-2/37), or bcl-2 deleted of the BH4 domain (BH4 del/8, BH4 del/42). (a) Values are expressed as means±S.D. of fold induction versus control cells (puro) exposed to normoxia. Results represent the average±S.D. of three independent experiments. (b and c) Western blots representative of three independent experiments with similar results are shown. β-actin and HSP70 proteins are shown as loading and transfering control. (d) Densitometric analysis of the HIF-1α protein level showed in western blot (c) was performed using Molecular Analyst Software and normalized to β-actin expression. Values are expressed as percentage of HIF-1α protein after CHX treatment versus HIF-1α protein expression in untreated cells (100%). (e) Western blot analysis of HIF-1α ubiquitination on control, bcl-2 wt and bcl-2 deleted of the BH4 domain cells after exposure to hypoxia. Whole cell lysates were immunoprecipitated (IP) with anti-HIF-1α antibody and then western blot analysis was performed using anti-ubiquitin antibody