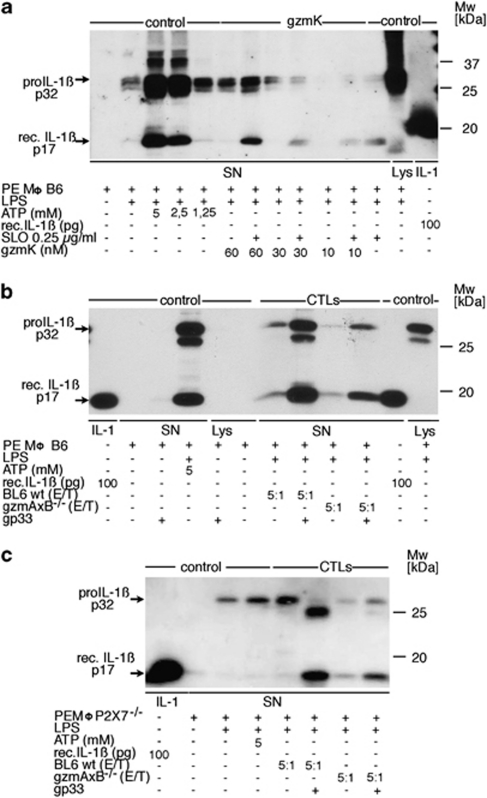
Figure 3.
(a) Induction of IL-1β release by rec. (Mo)gzmK. Adherent B6 PEMØs, previously sensitized with thioglycollate in vivo and challenged with LPS in vitro were incubated with the indicated amounts of gzmK in presence or absence of 0.25 μg/ml SLO, or ATP as positive control, for 3 h and SNs were subjected to WB analysis using anti-IL-1β-specific Ab. Aliquots of the following preparations were used as standards for WB analysis: SNs of ATP-sensitized pre-activated B6 PEMØs; SNs of thioglycollate-pre-sensitized and LPS-challenged B6 PEMØs. (b) Induction of IL-1β release by ex vivo CD8 cells expressing gzmK. Thioglycollate-pre-sensitized and LPS-challenged adherent B6 PEMØs were incubated with ex vivo-derived LCMV-immune Tc cells from B6 or gzmAxB ko mice at an E/T ratio of 5 : 1 in the presence or absence of the LCMV-specific peptide gp33. SN was taken after 6 h and subjected to WB analysis using anti-IL-1β-specific Ab. As control, SN from thioglycollate-pre-sensitized and LPS-challenged B6 PEMØs, and subsequently stimulated with 5 mM ATP, was analyzed accordingly. Aliquots of the following preparations were used as standards for WB analysis: rec. IL-1βs, p17; SN of ATP-sensitized pre-activated wt B6 PEMØs; SN of wt thioglycollate-pre-sensitized and LPS-activated B6 PEMØs. (c) Induction of IL-1β release by ex vivo CD8 cells expressing gzmK by targets lacking the P2X7 receptor. Experimental procedure was conducted as described in panel b, but with macrophages from P2X7−/− mice. Controls were prepared individually for this experiment with P2X7−/− macrophages (SN of ATP-sensitized pre-activated PEMØs; SN of wt thioglycollate-pre-sensitized and LPS-activated PEMØs)