Figure 8.
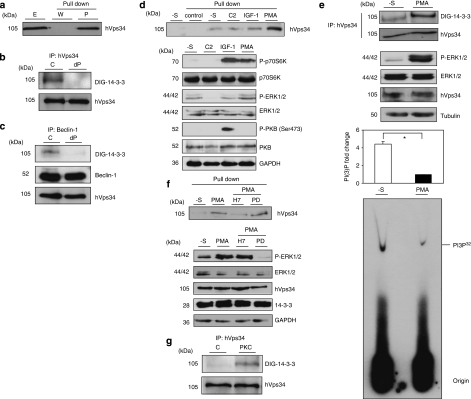
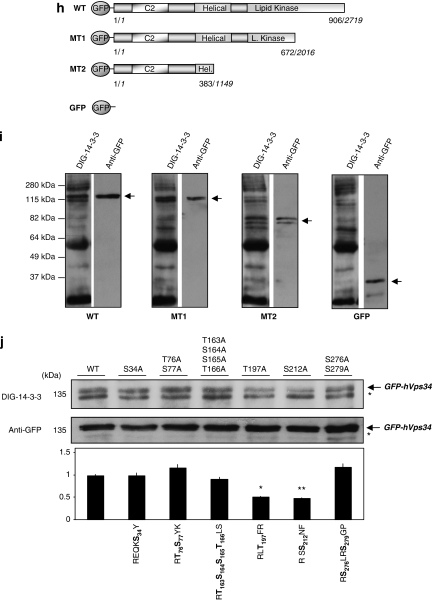
hVps34 binds 14-3-3 proteins by a phosphorylation-dependent mechanism. (a) HeLa cells continuously grown in the presence of serum were harvested as indicated and cell extracts (5 mg of protein) were incubated for 2 h with 50 μl of 14-3-3-Sepharose. Extract (E; 30 μg) and pellets, washed (W) and extracted with SDS-sample buffer (P), were run on SDS/PAGE and probed with indicated antibody. Serum-grown HeLa cell extract (1 mg) was incubated with 1 μg of hVps34 (Echelon Biosciences) (b) or Beclin-1 (Santa Cruz Biotechnology, Inc.) (c) antibodies bound to protein-G-Sepharose. The washed immunoprecipitates were used for dephosphorylation assay in the absence (C) or presence (dP) of 50 mU/ml PP2B and 15 mU/ml PP1 for 30 min at 30°C. Dephosphorylation was stopped using sample buffer and immunoprecipitates were resolved in SDS-PAGE, transferred to nitrocellulose and probed with DIG-14-3-3 (14-3-3 overlay), Beclin-1 (BD Biosciences) and hVps34 (Zymed) antibodies. (d) HeLa cells were treated with C2-ceramide (10 μM) for 24 h or serum-starved for 16 h (-S) and stimulated with IGF-1 (100 ng/ml, 20 min) or PMA (400 ng/ml, 15 min) as indicated. Cell extracts (5 mg of protein) were incubated for 2 h with 50 μl of 14-3-3-Sepharose according to Material and Methods and washed pellets (pull down) were extracted with SDS sample buffer, run on SDS/PAGE and probed with indicated antibody. Cell extract (30 μg) were run on SDS/PAGE transferred to nitrocellulose membranes and probed with indicated antibodies (bottom panel). (e) HeLa cells were serum starved for 16 h and left untreated (-S) or stimulated with PMA (400 ng/ml, 15 min). Cell extracts (1 mg) from both samples were incubated with 1 μg of hVps34 (Echelon Biosciences) antibody bound to protein-G-Sepharose. The washed immunoprecipitates were used for hVps34 in vitro kinase assay and also resolved to detect hVps34 protein and interaction with 14-3-3 analyzing with DIG-14-3-3. Cell extracts (30 μg) from both samples were run on SDS-PAGE, transferred to nitrocellulose membranes and probed with indicated antibodies (middle panel; quantification of hVps34 in vitro kinase assay expressed as PI(3) fold change, N=3, *P=0.0021, Student t-test). (f) HeLa cells grown in medium containing serum were serum-starved for 16 h (-S). Where indicated, cells were incubated with H-7 (100 μM), PD 184352 (2 μM) or no inhibitor for 1 h before stimulation with PMA (400 ng/ml) for a further 15 min. Cell extracts (5 mg) were incubated for 2 h with 50 μl of 14-3-3-Sepharose and washed pellets were extracted with SDS sample buffer, run on SDS/PAGE and probed with indicated antibody. Cell extract (30 μg) were run on SDS/PAGE transferred to nitrocellulose membranes and probed with indicated antibodies (bottom panel). (g) hVps34 was immunoprecipitated from HeLa cells that had been serum-starved for 16 h. The hVps34 immunoprecipitates were incubated in the presence of catalytic subunit of PKC (1 U/ml rat brain PKC) with MgATP for 30 min at 30°C, and analyzed by DIG-14-3-3 overlays. The lane marked C is a control with no PKC. (h) Schematic representation of hVps34 protein structure. N-terminal region of ∼50 amino acids, followed by C2 domain probably related to acidic phospholipids interactions, a possible regulatory helical domain and kinase domains with the extreme C-terminal 11 residues required for lipid kinase activity (26, 27). (i) GFP-tagged fragments of hVps34 were constructed: WT contains GFP-tagged full-length hVps34 (906 aa, 2719 pb), GFP-tagged fragment MT1 lost the residues required for hVps34 kinase activity and GFP-tagged fragment MT2 just contain the N-terminal part of the protein. GFP-tagged fragment of hVps34 were transiently expressed in HEK293T cells stimulated with PMA. GFP-tagged sequences were immunoprecipitate using anti-sheep GFP antibodies and carried out an in vitro binding reactions using DIG-14-3-3 overlay assay. Arrows show the correspondence GFP-tagged fragment reveal by anti-rabbit GFP antibody. (j) HEK293T cells were transfected with plasmid expressing GFP-hVps34 WT and several GFP-hVps34 mutants as indicated. After 24 h, cells were serum-starved for a further 16 h, then stimulated for 15 min with PMA (400 ng/ml). Cell extracts (0.5 mg) were incubated with 10 μg of anti-sheep GFP antibody bound to protein-G-Sepharose. The washed immunoprecipitates were used to detect GFP-hVps34 protein and its interaction with 14-3-3 proteins was analyzed by DIG-14-3-3 (bottom panel: quantification of DIG-14-3-3 signals correspond to GFP-hVps34 immunoprecipitates expressed as arbitrary unit, N=3, *P=0.0003, **P=0.0008, Student t-test)