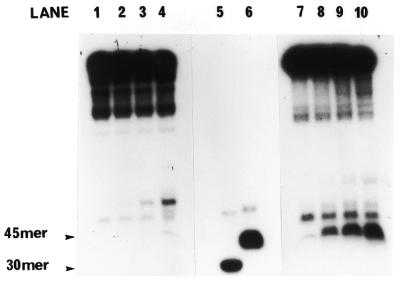
Figure 5.
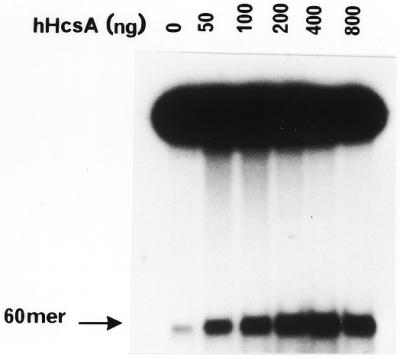
In vitro helicase activity of hHcsA expressed in E.coli. (A) A standard helicase assay was carried out utilizing a 50 bp duplex substrate and increasing amounts of purified hHcsA (fraction V). Inhibition at higher protein concentrations was presumably due to salt. (B) Analysis of the polarity of translocation. A time-course analysis of helicase activity (100 ng HcsA fraction III) using the directionality substrate, as described in Materials and Methods, was carried out in the presence of 250 ng RPA. Lanes 1–5 correspond to assay points in which the 30mer duplex is 5′-end-labeled, while lanes 6–10 correspond to assay points in which the 45mer duplex is 3′-end-labeled. Lane 1, 0 min; lane 2, 1 min; lane 3, 2 min; lane 4, 5 min; lane 5, heat denatured substrate labeled at the 30mer duplex end; lane 6, heat denatured substrate labeled at the 45mer duplex end; lane 7, 0 min; lane 8, 1 min; lane 9, 2 min; lane 10, 5 min.