Abstract
Up to 14% of Malawian adults die during the intensive phase of tuberculosis treatment. In a prospective cohort of 199 Malawian adults with microbiologically confirmed pulmonary tuberculosis, clinical and laboratory parameters were compared between those who died or deteriorated with those who had an uneventful recovery. Baseline tumor necrosis factor alpha responses to stimulation with heat-killed Mycobacterium tuberculosis and lipopolysaccharide were reduced among the 22 patients with poor outcome (P = .017). Low body mass index (P = .002) and elevated respiratory rate (P = .01) at tuberculosis diagnosis independently predicted poor outcome. Validation of a clinical score identifying high-risk individuals is warranted, together with further investigation of immunological derangements.
In Malawi, up to 14% of tuberculosis (TB) patients die during the 2-month intensive phase of therapy [1]. Although cotrimoxazole prophylaxis and antiretroviral therapy (ART) have been increasingly available since 2003 for the two-thirds of TB patients who are coinfected with human immunodeficiency virus (HIV), these have not significantly reduced early mortality [2].
Clinical deterioration [3] and poor outcome [4] have been associated with elevated serum tumor necrosis factor alpha (TNF-α), a pivotal proinflammatory cytokine. While sufficient production is necessary for an adequate host immune response, excessive levels are associated with immunopathology [5]. Many mycobacterial components provoke a proinflammatory response [6]. Dysregulated proinflammatory responses may be more pronounced in patients coinfected with TB and HIV; HIV causes innate immune activation [7], whereas depletion of CD4 cells reduces interferon (IFN)-γ production [8]. We hypothesized that a proportion of patients deteriorate clinically or die during the initial phase of TB treatment as a consequence of a proinflammatory process.
METHODS
Patient Selection
Ambulatory and hospitalized adults with newly diagnosed pulmonary tuberculosis (PTB) were recruited 2 mornings per week at Queen Elizabeth Central Hospital, Blantyre, Malawi. The resulting enrollment of 321 patients was a subset of the 3800 PTB cases registered between February 2007 and February 2009.
Exclusion criteria were: age <16 years; known pregnancy; previous TB; clinical evidence of extrapulmonary TB; and in HIV-infected patients, already receiving ART.
Ethical approval for this study was granted by the College of Medicine Research Ethics Committee, University of Malawi, and the ethics committee of the Liverpool School of Tropical Medicine.
Clinical Assessment
Under routine conditions in Blantyre, most patients commence and complete treatment without further clinical assessment or investigation. By contrast, study patients received additional input, described below.
Prior to commencing treatment, a standardized assessment involving detailed history, examination, and chest radiography was undertaken. Full blood count (Coulter Hmx Hematology Analyzer), CD4 count (Becton Dickinson FACScount) and HIV testing (both Determine HIV 1/2 kit and Uni-Gold HIV-1/2 kit; tie-breaker, SD Bioline HIV 1/2 kit) were performed. Three additional sputum samples were collected for microbiological confirmation of the routine laboratory diagnosis. Patients were defined as smear positive if at least 1 acid-fast bacillus was detected in at least 1 sputum sample by Ziehl--Neelsen staining [9] and culture positive in accordance with standard methods [10].
Patients were reviewed on days 3, 7, 28, and 56 of TB treatment. Additionally, patients were asked to present at any time if they felt acutely unwell. This was considered an “acute episode”; full assessment was followed by investigation and management as clinically indicated and feasible within our setting. An episode was considered potentially life-threatening if hospitalization and intensive medical input were required. The cause of each acute episode or death was determined by 2 of the authors (CJW and NPKB); discussion with a third clinician took place in the single event of disagreement.
Patients who defaulted follow-up were traced in the community and the vital status of the patient was determined when found. Consistent with national policy, eligible patients commenced ART after the intensive phase of TB treatment.
Whole Blood Assay
The whole blood method described by Weir et al [11] was used. Heparinized whole blood was diluted 1:5 with serum-free media (RPMI 1640 medium, 2 mM l-glutamine, 100 IU/mL penicillin/100 μg/mL streptomycin [all Sigma-Aldrich]). Quadruplicates of 250 μL diluted blood were added to a 96-well tissue culture plate (Fisher Scientific) and stimulated with 5 × 106 cfu/mL heat-killed Mycobacterium tuberculosis H37Rv (HK) (a gift from Dr R Hartkoorn, University of Liverpool), .01 μg/mL Escherichia coli, strain 055.b5 lipopolysaccharide (LPS) (Sigma-Aldrich), or 5 μg/mL phytohemagglutinin (PHA) (Sigma-Aldrich L8902); a negative control of unstimulated blood was included. After 24 hours (HK and LPS) or 6 days (HK and PHA) incubation in 5% CO2 at 37°C, supernatants from quadruplicate wells were harvested and stored at –80°C until further analysis.
Cytokine Measurement
TNF-α and IFN-γ were analyzed from stored supernatants after clinical categorization of acute episodes by enzyme-linked immunosorbent assay using antibody pairs and recombinant cytokine standards, according to the manufacturer’s protocols (BD Pharmingen). The lower limit of detection was 50 pg/mL in both assays. Response to each antigen was calculated by subtracting the value obtained from unstimulated blood.
Statistical Analysis
It was considered that without intervention, patients with life-threatening acute episodes would likely have died. These patients were therefore grouped a priori together with those who died as “poor outcome” for comparison with “good outcome” patients who had an uncomplicated clinical course.
Associations between potential risk factors and poor outcome were explored using univariate logistic regression. Variables with P < .10 were included in backward multiple logistic regression, being retained in the model at a significance level of .05. Likelihood ratio tests were used to test significance.
Mann–Whitney U tests were used to compare median cytokine levels (GraphPad Prism version 5.00 for Windows, GraphPad Software)
RESULTS
Description of Clinical Cohort
Of 321 patients enrolled, 221 (69%) had microbiologically proven TB. Two patients were withdrawn due to ineligibility, and 17 elected to withdraw. Ten percent of patients defaulted; in all but 3 patients, it was verified that the patient survived the study period, either by locating the patient or by consulting the “cured” or “treatment completed” outcome category from the TB registry. These were analyzed as protocol deviations. Primary analysis was performed on the 199 patients with proven TB who remained in the study. Sixty-one percent were male, 72% were sputum smear positive, and 60% were HIV positive with a median CD4 count of 150 (IQR, 68–346) cells/mm3. Fifteen (7.5%) were hospitalized at the time of TB diagnosis.
Description of Deaths and Acute Episodes
Twelve patients (6%) died during the study period after a median of 12 days; 8 during a hospital admission and 4 at home. Eighty-three percent were HIV positive. Two patients had self-presented with bacterial pneumonia (diagnosis based on symptoms, clinical signs, and new lobar infiltrate on chest x-ray) and failed to respond to aggressive fluid and antibiotic therapy. Two patients developed a septic shock–like presentation, 1 of whom had Salmonella typhimurium isolated from the blood. Six patients (50% of deaths) were thought to have died of tuberculosis itself, and death was attributed to TB or another HIV-related pathogen in the final 2.
Sixteen patients (8%) presented with an acute episode after a median of 18 days. Twelve of these were considered to be potentially life threatening, requiring hospitalization. Sixty percent of patients were HIV positive. In 50% of HIV-negative and 63% of HIV-positive patients, the cause of self-presentation with a life-threatening acute episode was a presumed superadded infection. Following intervention, 10 (83%) recovered clinically; 2 HIV-positive patients with superadded bacterial pneumonia died (described above as deaths). Two patients with minor skin rashes, 1 with peripheral neuropathy, and 1 with a transient confusional state that resolved spontaneously were not deemed severe.
The event rate in the patients without microbiologically confirmed disease (14 of 100) was not significantly different (X2, 1.13; P = .29).
Baseline Clinical Factors Predicting Life-Threatening Acute Episode or Death
Reduced systolic blood pressure, O2 saturation, body mass index (BMI), hemoglobin, platelet count, CD4 count, and increased respiratory rate and pulse were each significantly associated with poor outcome on univariate analysis (Table 1). Notably, there was no significant association with HIV status, duration of symptoms, or whether the patient was an inpatient at the time of TB diagnosis.
Table 1.
Univariate Analysis of Risk Factors for Acute Episode or Death
| Variable | Median (range) or number in category | Odds ratio | 95% CI | P value |
| Demographic Factors (OR is relative to nontabulated category) | ||||
| Age >35 | 29 (15–70) | 1.63 | .64–4.13 | .31 |
| Female sex | n = 76 | 1.74 | .65–4.67 | .25 |
| Clinical History and Examination | ||||
| Categorical Variables (OR is relative to nontabulated category) | ||||
| HIV positive (known or index presentation) | n = 120 | 1.74 | .65–4.68 | .26 |
| Reported weight loss | n = 171 | 3.78 | .49–29.3 | .12 |
| Shortness of breath | n = 146 | 1.69 | .54–5.24 | .34 |
| Ever smoked | n = 50 | .91 | .31–2.65 | .86 |
| Inpatient at enrollment | n = 15 | 3.00 | .75–12.00 | .15 |
| Bilateral chest signs | n = 30 | 1.97 | .66–5.91 | .24 |
| Continuous Variables (OR is for the increase or decrease stated) | ||||
| Duration of cough (per week) | 6 (1–56) | .99 | .94–1.04 | .71 |
| Performance status (0–4)a | 1 (0–4) | 1.60 | .98–2.61 | .07 |
| Reduced BMI (per 1 kg/m2) | 18.5 (12.3–35.3) | 1.53 | 1.19–1.96 | <.001d |
| Increased temperature (per 1°C) | 37.4 (34–40.2) | .92 | .64–1.34 | .68 |
| Reduced O2 saturations (per 5% decrease) | 96 (60–100) | 1.72 | 1.09–2.71 | .01d |
| Increased respiratory rate (per 5 breaths/min) | 16 (2–48) | 1.82 | 1.16–2.85 | .01d |
| Increased pulse (per 10 beats/min)b | 105 (20–160) | 1.26 | 1.01–1.57 | .04d |
| Reduced systolic blood pressure (per 5 mm Hg) | 100 (60–160) | 1.28 | 1.00–1.64 | .04d |
| Disorientation (0–3: time, place, or person) | 3 (0–3) | 2.31 | .89–6.01 | .10 |
| Baseline Investigations | ||||
| Categorical Variables | ||||
| CD4 <200 (HIV-positive patients only)c | n = 64 | 3.67 | .98–13.70 | .03d |
| Sputum smear negative | n = 110 | 1.80 | .68–4.80 | .24 |
| Chest x-ray changes atypical for TB | n = 58 | 1.01 | .35–2.88 | .99 |
| Continuous Variables (presented for a unit increase or decrease unless otherwise stated) | ||||
| Reduced hemoglobin (g/dL) | 9.9 (4.1–16.5) | 1.38 | 1.09–1.75 | .01d |
| Total white cell count (109/L) | 6.8 (1.3–21.7) | .85 | .69–1.04 | .08 |
| Monocyte count (109/L) | 0.6 (01–5.1) | .83 | .30–2.30 | .70 |
| Reduced platelet count (per 10 × 109/L) | 358 (18–928) | 1.04 | 1.01–1.08 | .01d |
| Number of zones involved on chest x-ray | 1 (0–6) | 1.33 | .93–1.90 | .10 |
NOTE. BMI, body mass index; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; TB, tuberculosis.
de Valliere S, Barker RD. Poor performance status is associated with early death in patients with pulmonary tuberculosis. Trans R Soc Trop Med Hyg 2006;100:681–6.
Not significant if patients lost to follow-up were considered to have suffered an adverse event.
Not included in multiple logistic regression as only relevant for HIV-positive patients.
Included in initial multivariate model.
A backward stepwise approach for 175 patients with data on all included variables identified low BMI (odds ratio [OR], 1.44; 95% confidence interval [CI], 1.11–1.86; P = .002) and elevated respiratory rate (OR, 1.81; 95% CI, 1.14–2.87; P = .01 per 5 breaths/min) as independent risk factors. Low BMI was significantly associated with poor outcome in both HIV-positive and HIV-negative patients.
Cytokine Production Using Whole Blood Assay
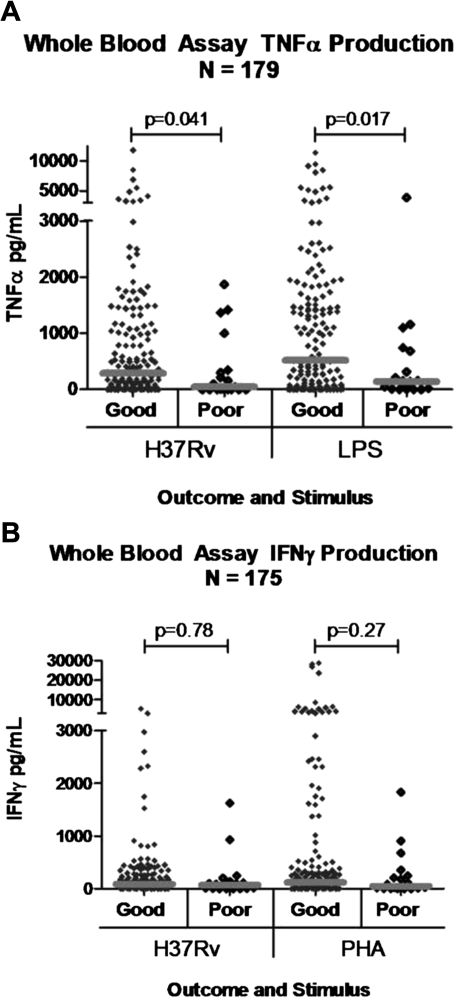
At the time of enrollment, in response to stimulation of whole blood with both heat-killed H37Rv and LPS, there was significantly lower TNF-α production in patients with poor outcome (median, 47 pg/mL [IQR, 0–346] in response to H37Rv and 142 pg/mL [IQR, 11–683] in response to LPS) compared with good outcome (median, 290 pg/mL [IQR, 29–1116] in response to H37Rv [P = .041] and 522 pg/mL [IQR, 58–1619] in response to LPS [P = .017]) (Figure 1a). As a predictor of poor outcome, ORs for deterioration were 1.72 (95% CI, .78–3.79; P = .09) and 1.87 (95% CI, .94–3.73; P = .02) for each decrement of 1000 pg/mL TNF-α produced in response to heat-killed TB and LPS, respectively, for univariate analysis; neither was statistically significant for multivariate analysis (P > .1). There was no significant difference in TNF-α from unstimulated blood (data not shown). The trend was apparent in both HIV-positive and HIV-negative individuals (data not shown); in the HIV-positive subset, TNF-α production and CD4 count (data not shown) were not correlated. Intracellular cytokine staining showed the monocyte to be the major source of TNF-α (data not shown); monocyte count did not differ between the groups. TNF-α production and BMI were not associated either at baseline or following recovery.
Figure 1.
Scattergraphs (and medians) of cytokine production in response to stimulation of whole blood with heat-killed H37Rv, lipopolysaccharide (LPS), and phytohemagglutinin (PHA) immediately prior to the start of tuberculosis (TB) treatment. Patients who died or suffered a significant acute episode are grouped together under ”poor outcome” whereas patients who had an uncomplicated clinical course were considered to have a ”good outcome.” A, Production of tumor necrosis factor alpha (TNF-α) after 24 hours incubation. B, Production of interferon (IFN)-γ after 6 d incubation.
Analysis of IFN-γ production in response to stimulation with both heat-killed H37Rv and PHA revealed no significant differences between outcome groups (Figure 1b).
DISCUSSION
This prospective cohort study investigated the causes, timing, and risk factors for clinical deterioration and death during the intensive phase of TB treatment in Malawi.
Independent predictors for poor outcome were low BMI and elevated respiratory rate. Detailed clinical evaluation by experienced clinicians yielded no additional benefit in determining risk. Simple clinical scoring systems enable triage and prioritization of medical admissions [12] but have not been studied in relation to TB. Validation of a clinical score to predict risk in TB patients is necessary in a larger cohort.
Using clinical criteria, half of the deaths in our study were attributed to tuberculosis, and one-third may have resulted from intercurrent bacterial infection, although this was not universally proven microbiologically. Presumed bacterial infection was the most common etiology for clinical deterioration not leading to death, occurring with equal frequency in HIV-positive and HIV-negative patients. All HIV-positive patients were receiving cotrimoxazole prophylaxis in accordance with national policy and Joint United Nations Programme on HIV/AIDS guidelines. Knowledge of HIV status did not aid prediction of adverse outcome during the intensive phase of TB treatment, directly contrasting with findings from South Africa [13].
Significantly lower TNF-α production occurred in response to both TB and LPS in patients who subsequently died or suffered significant clinical deterioration, irrespective of HIV status, contrary to our hypothesis. A similar finding emerged from investigation of 41 Zambian TB patients, where 7 HIV-positive patients died [14] but clinical predictors of poor outcome were not identified.
Lower monocyte TNF-α production and the propensity for bacterial infections among these patients suggest impaired innate immune defenses. Monocyte dysfunction occurs in patients with septic shock [15], predisposing to secondary bacterial infection, which can be overwhelming. An analogous process, not previously described in tuberculosis, may explain our findings; work is ongoing to elucidate this process. Furthermore, cytokine networks are increasingly recognized as pivotal to the outcome of infectious diseases. Additional investigation of this Malawian cohort will further compare the cytokine milieu in patients with poor outcome to those with an uncomplicated clinical course.
There are some limitations to this study. No prior data associated baseline TNF-α responses with clinical outcome, making sample size calculation impossible. This is among the largest longitudinal studies evaluating induced cytokine responses in TB patients. However, sample size was a limiting factor in the development of a predictive model, and validation in a larger cohort is needed. Limited diagnostic resources are available in Malawi. Nonetheless, following appropriate treatment, most patients with presumed superadded bacterial infection recovered, suggesting that our diagnoses were correct.
CONCLUSIONS
Patients at risk of clinical deterioration after starting TB treatment are identifiable using simple measurements including BMI and respiratory rate. Further clinical studies should seek to validate a simple clinical score for prediction of risk in TB patients. Superadded bacterial infection is an important cause of deterioration, and is associated with depressed monocyte responses to antigenic stimulation. Further work should focus on the mechanisms underlying these impaired innate responses, as this may guide improved therapeutic and predictive strategies.
Funding
C. J. W. is supported by a Wellcome Trust Training Fellowship (GR076210MA), B. K. is supported by a Senior National Institute for Health Research (NIHR) Research Fellowship, and M. L. W. is supported by a Programme Grant from the Wellcome Trust held by R. S. H. M. P. is an NIHR senior investigator and acknowledges the support of the Department of Health (National Health Service Chair of Pharmacogenetics), Medical Research Council (MRC Centre for Drug Safety Science), European Commission-FP7, and Wellcome Trust.
Acknowledgments
We thank the patients and staff of Queen Elizabeth Central Hospital, Blantyre, Malawi, and the National TB Control Programme. We thank the Core Laboratory team at the Malawi-Liverpool-Wellcome Clinical Research Programme, supervised by Mike Moore, for the performance of diagnostic microbiological and hematological investigations. Finally, we thank the members of the study team: George Musowa, Enid Mfungwe, Bernard Kasinja, Suzgo Nyirongo, and George Daza.
References
- 1.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–5. [PubMed] [Google Scholar]
- 2.Zachariah R, Fitzgerald M, Massaquoi M, et al. Does antiretroviral treatment reduce case fatality among HIV-positive patients with tuberculosis in Malawi? Int J Tuberc Lung Dis. 2007;11:848–53. [PubMed] [Google Scholar]
- 3.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–4. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh SM, Hung CC, Chen MY, Sheng WH, Chang SC. Dynamics of plasma cytokine levels in patients with advanced HIV infection and active tuberculosis: implications for early recognition of patients with poor response to anti-tuberculosis treatment. AIDS. 1999;13:935–41. doi: 10.1097/00002030-199905280-00009. [DOI] [PubMed] [Google Scholar]
- 5.Mootoo A, Stylianou E, Arias MA, Reljic R. TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets. 2009;8:53–62. doi: 10.2174/187152809787582543. [DOI] [PubMed] [Google Scholar]
- 6.Al-Attiyah R, Mustafa AS, Abal AT, El-Shamy AS, Dalemans W, Skeiky YA. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:139–44. doi: 10.1111/j.1365-2249.2004.02609.x. PMCID: 1809193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bal AM, Lakhashe SK, Thakar MR, Tripathy SP, Paranjape RS. Dysregulation of proinflammatory and regulatory cytokines in HIV infected persons with active tuberculosis. Cytokine. 2005;30:275–81. doi: 10.1016/j.cyto.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. PMCID: 330075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, ed. Seventh meeting of the WHO Strategic and Technical Advisory Group for Tuberculosis (STAG-TB). 2007. World Health Organisation. http://www.who.int/tb/events/stag_report_2007.pdf. Accessed 10 May 2011. [Google Scholar]
- 10.World Health Organization. Laboratory services in tuberculosis control. Part 3: culture. 1998. World Health Organisation. http://wwwn.cdc.gov/dls/ila/documents/lstc3.pdf. Accessed 10 May 2011. [Google Scholar]
- 11.Weir RE, Brennan PJ, Butlin CR, Dockrell HM. Use of a whole blood assay to evaluate in vitro T cell responses to new leprosy skin test antigens in leprosy patients and healthy subjects. Clin Exp Immunol. 1999;116:263–9. doi: 10.1046/j.1365-2249.1999.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94:521–6. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 13.Pepper DJ, Rebe K, Morroni C, Wilkinson RJ, Meintjes G. Clinical deterioration during antitubercular treatment at a district hospital in South Africa: the importance of drug resistance and AIDS defining illnesses. PLoS One. 2009;4:e4520. doi: 10.1371/journal.pone.0004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedland JS, Hartley JC, Hartley CG, Shattock RJ, Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–8. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–83. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]