Endothelium-derived nitric oxide (NO) is vasoprotective, as it enhances endothelial cell survival and proliferation, inhibits the excessive proliferation of vascular smooth muscle cells, and suppresses the adhesion of platelets and inflammatory cells to the vessel wall [1]. Substantial evidence from pre-clinical studies and human research indicates that impairment of the endothelial NO synthase (NOS) pathway accelerates vascular disease, and increases the risk for major adverse cardiovascular events [2–5]. Impairment of the NOS pathway is multifactorial, but it is increasingly apparent that circulating inhibitors of NOS play an important role. Asymmetric dimethylarginine (ADMA) and monomethyl-L-arginine (MMA) [6] are endogenous competitive inhibitors of NOS. Most human studies have focused on ADMA, as it is the more prevalent species in human plasma. Plasma ADMA is elevated in patients with cardiovascular disease or with risk factors, and it contributes to vascular resistance and stiffness [7, 8]. Notably, several large studies have shown that plasma ADMA is an independent biomarker for cardiovascular morbidity and total mortality [4, 5, 9]. Accordingly, endogenous mechanisms that regulate ADMA are deserving of further scientific attention.
Synthesis and Metabolism of ADMA
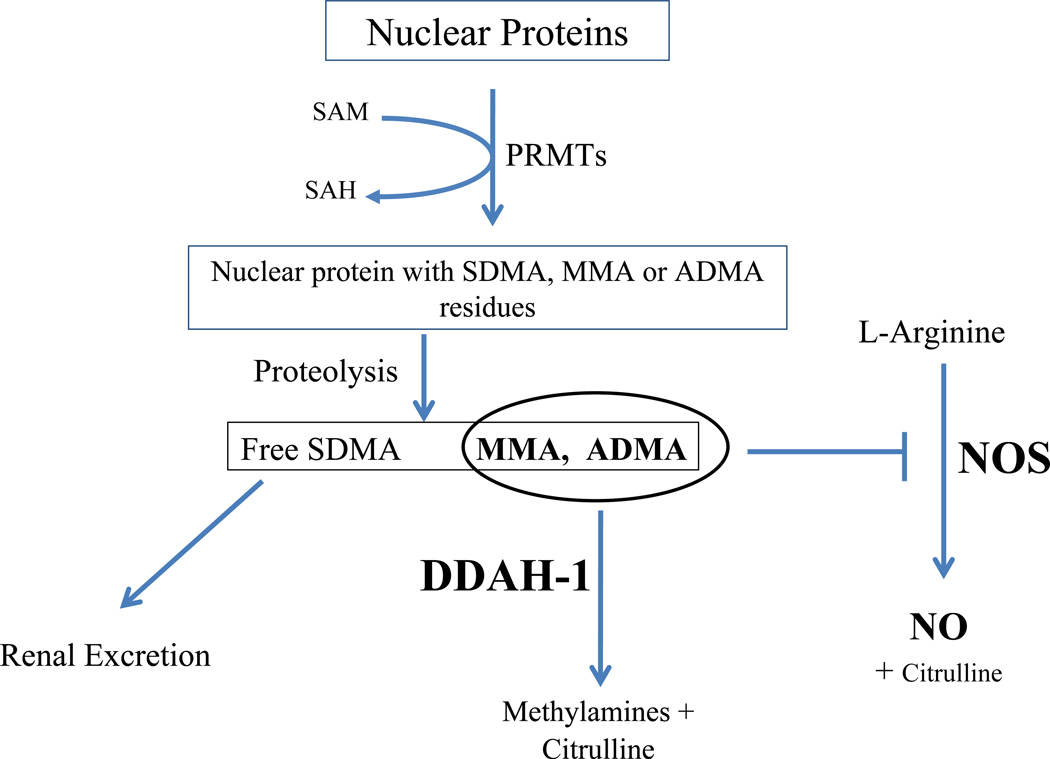
Protein-arginine methyltransferases (PRMTs) methylate arginine residues on histone and other nuclear proteins [10–12]. When these proteins are hydrolyzed, free methylarginines are released, including ADMA, MMA, and symmetric dimethylarginine (SDMA; this latter methylarginine does not inhibit NOS) (Figure). These may be expelled from the cell by the cationic (CAT) transporter to be secreted in the urine, which is the primary route for SDMA clearance. However, the majority of ADMA and MMA (~80%) is degraded within the cell by dimethylarginine dimethylaminohydrolase (DDAH) [13–16]. The activity of DDAH is reduced by oxidative stress that is associated with cardiovascular disease [17–19], causing ADMA levels to become elevated in these conditions [13, 20]. By contrast, global overexpression of DDAH1 in transgenic mice reduces ADMA levels and increases NO production [21–23]. These DDAH-1 overexpressing mice manifest reduced vascular resistance, increased insulin sensitivity and enhanced endothelial regeneration [21–23]; and they are resistant to vascular lesion formation induced by endothelial denudation, vascular inflammation, or hypercholesterolemia [22, 24, 25]. These observations are consistent with an essential role of DDAH1 in maintaining vascular homeostasis.
Figure.
The role of DDAH1 in the metabolism of the NOS antagonists ADMA and MMA levels. DMA = dimethylamine. PRMTs = protein arginine methyltransferases. Other abbreviations as in text.
Global DDAH1 Deletion: Accumulation of ADMA and Loss of Homeostasis
In this issue of ATVB, Hu and colleagues [26] provide strong evidence that, of the two DDAH isoforms (DDAH1 and DDAH2), DDAH1 is largely responsible for the degradation of ADMA. They generated a murine model of global DDAH1 knockout (DDAH1−/−) by targeting exon4 of the DDAH1 gene. The DDAH1−/− mice displayed normal developmental features while showing negligible tissue DDAH activity in several tissues. The abrogation of DDAH enzymatic activity (assessed using isotope-labeled ADMA or MMA) was surprising, since expression of the DDAH-2 isoenzyme was unaffected. The expression of endothelial NOS (eNOS), PRMTs 1 and 3, and CAT were unaffected also. With the loss of DDAH activity there were significant elevations in tissue and plasma ADMA and MMA.
Isolated aortic rings from the DDAH1−/− mice manifested impaired endothelium dependent vasodilation in response to acetylcholine, consistent with ADMA-induced suppression of NOS. These animals also exhibited a significant increase in blood pressure, reminiscent of the hemodynamic abnormality in eNOS knockout mice [27]. The elevation in BP was reversed by infusion of L-arginine consistent with the competitive inhibition of NOS by ADMA.
This study confirms the importance of DDAH in regulating NO synthesis, by its degradation of the endogenous NOS inhibitors ADMA and MMA. Furthermore, this study suggests that a specific isoenzyme, DDAH1, is primarily responsible for metabolism of the methylarginines, and that DDAH2 cannot compensate for the loss of DDAH1.
Future Directions
Although the study of Hu and coworkers [26] complements previous studies using the endothelial-specific DDAH1 knockout and heterozygous DDAH1 deficient mice [28, 29], it also raises some interesting questions. Firstly, there is a discrepancy between this study and a previous one which suggested that the global DDAH-1 knockout was lethal [29]. It is possible that in the previous study (in which exon1 of DDAH was targeted), the deletion might have adversely affected another genomic region necessary for embryogenesis.
If DDAH2 does not compensate for the loss of DDAH1, what may be its function? The literature is mixed regarding the importance of DDAH2 in the metabolism of ADMA [6, 30–32]. Overexpression of DDAH-2 improves endothelium dependent vasorelaxation and increases NO synthesis, whereas siRNA knockdown of DDAH-2 reduces NO synthesis [31–33] However, the story becomes more interesting by recent evidence that both DDAH-1 and DDAH-2 manifest protein-protein interactions that may affect the NOS pathway independently of ADMA metabolism [31, 34]
The global DDAH-1 knockout mouse of Hu and colleagues will be useful to further interrogate the role of DDAH1 deficiency in vascular disorders. In the meanwhile, the weight of the evidence indicates that DDAH-1 is a worthy therapeutic target. Agents which increase DDAH expression are known [35, 36] and one of these, an FXR agonist, is in clinical trials [37]. An alternative approach is to develop an allosteric activator of the enzyme. Although development of an allosteric activator is not a typical pharmaceutical approach, recent studies indicate that this may be an achievable aim [38, 39]. An agent that increases the expression and/or activity of DDAH-1 would be anticipated to reduce blood pressure, enhance insulin sensitivity, and reduce adverse cardiovascular outcomes.
Acknowledgements
This work was supported in part by grants from the NIH (RC2HL103400, 1U01HL100397, and K12HL087746), AHA (11IRG5180026), Stanford SPARK Program and by the Tobacco-Related Disease Research Program of the University of California (18XT-0098).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooke JP, Ghebremariam YT. Dietary nitrate, nitric oxide and restenosis. J Clin Invest. 2011;121:1258–1260. doi: 10.1172/JCI57193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niebauer J, Maxwell AJ, Lin PS, Wang D, Tsao PS, Cooke JP. NOS inhibition accelerates atherogenesis: reversal by exercise. Am J Physiol Heart Circ Physiol. 2003;285:H535–H540. doi: 10.1152/ajpheart.00360.2001. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99:1141–1146. doi: 10.1161/01.cir.99.9.1141. [DOI] [PubMed] [Google Scholar]
- 4.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson AM, Shin DS, Weatherby C, Harada RK, Ng MK, Nair N, Kielstein J, Cooke JP. Asymmetric dimethylarginine predicts adverse cardiovascular events and mortality in peripheral arterial disease. Vasc Med. 2010;15:267–274. doi: 10.1177/1358863X10364552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 7.Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, Hoeper MM, Haller H, Fliser D. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–177. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- 8.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Böger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–2029. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 9.Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, Minar E, Müller M, Wolzt M, Schillinger M. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–2540. doi: 10.1161/01.ATV.0000242801.38419.48. [DOI] [PubMed] [Google Scholar]
- 10.Aletta JM, Cimato TR, Ettinger MJ. Protein methylation: a signal event in post-translational modification. Trends Biochem Sci. 1998;23:89–91. doi: 10.1016/s0968-0004(98)01185-2. [DOI] [PubMed] [Google Scholar]
- 11.Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol Ther. 2007;113:50–87. doi: 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;16(33):1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109:1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 14.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 15.Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005;10:S73–S81. doi: 10.1191/1358863x05vm597oa. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-L-arginine to L-citrulline in rats. Biochem Biophys Res Commun. 1987;148:671–677. doi: 10.1016/0006-291x(87)90929-6. [DOI] [PubMed] [Google Scholar]
- 17.Weis M, Cooke JP. Cardiac allograft vasculopathy and dysregulation of the NO synthase pathway. Arterioscler Thromb Vasc Biol. 2003;23:567–575. doi: 10.1161/01.ATV.0000067060.31369.F9. [DOI] [PubMed] [Google Scholar]
- 18.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 19.Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 20.Abbasi F, Asagmi T, Cooke JP, Lamendola C, McLaughlin T, Reaven GM, Stuehlinger M, Tsao PS. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:1201–1213. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 21.Sydow K, Mondon CE, Schrader J, Konishi H, Cooke JP. Dimethylarginine dimethylaminohydrolase overexpression enhances insulin sensitivity. Arterioscler Thromb Vasc Biol. 2008;28:692–697. doi: 10.1161/ATVBAHA.108.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konishi H, Sydow K, Cooke JP. Dimethylarginine dimethylaminohydrolase promotes endothelial repair after vascular injury. J Am Coll Cardiol. 2007;49:1099–1105. doi: 10.1016/j.jacc.2006.10.068. [DOI] [PubMed] [Google Scholar]
- 23.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation. 2003;108:3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 24.Jacobi J, Maas R, Cardounel AJ, Arend M, Pope AJ, Cordasic N, Heusinger-Ribeiro J, Atzler D, Strobel J, Schwedhelm E, Böger RH, Hilgers KF. Dimethylarginine dimethylaminohydrolase overexpression ameliorates atherosclerosis in apolipoprotein E-deficient mice by lowering asymmetric dimethylarginine. Am J Pathol. 2010;176:2559–2570. doi: 10.2353/ajpath.2010.090614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Sydow K, Gunawan F, Jacobi J, Tsao PS, Robbins RC, Cooke JP. Dimethylarginine dimethylaminohydrolase overexpression suppresses graft coronary artery disease. Circulation. 2005;112:1549–1556. doi: 10.1161/CIRCULATIONAHA.105.537670. [DOI] [PubMed] [Google Scholar]
- 26.Hu X, Atzler D, Xu X, Zhang P, Guo H, Lu Z, Fassett J, Schwedhelm E, Böger RH, Bache RJ, Chen Y. Global Dimethylarginine Dimethylaminohydrolase-1 (DDAH1) Gene-Deficient Mice Reveal That DDAH1 Is the Critical Enzyme for Degrading the Cardiovascular Risk Factor Asymmetrical Dimethylarginine. Arterioscler Thromb Vasc Biol. 2011;(this issue) doi: 10.1161/ATVBAHA.110.222638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, Schwedhelm E, Lüneburg N, Böger RH, Zhang P, Chen Y. Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine. Circulation. 2009;120:2222–2229. doi: 10.1161/CIRCULATIONAHA.108.819912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 30.Feng M, Liu L, Guo Z, Xiong Y. Gene transfer of dimethylarginine dimethylaminohydrolase-2 improves the impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells induced by lysophosphatidylcholine. Eur J Pharmacol. 2008;584:49–56. doi: 10.1016/j.ejphar.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. J Biol Chem. 2009;284:35338–35347. doi: 10.1074/jbc.M109.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101:627–635. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 33.Torondel B, Nandi M, Kelly P, Wojciak-Stothard B, Fleming I, Leiper J. Adenoviral-mediated overexpression of DDAH improves vascular tone regulation. Vasc Med. 2010;15:205–213. doi: 10.1177/1358863X09360264. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Hu X, Xu X, Chen Y, Bache RJ. Dimethylarginine dimethylaminohydrolase 1 modulates endothelial cell growth through nitric oxide and Akt. Arterioscler Thromb Vasc Biol. 2011;31:890–897. doi: 10.1161/ATVBAHA.110.215640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Wilson A, Gao X, Kuruba R, Liu Y, Poloyac S, Pitt B, Xie W, Li S. Coordinated regulation of dimethylarginine dimethylaminohydrolase-1 and cationic amino acid transporter-1 by farnesoid X receptor in mouse liver and kidney and its implication in the control of blood levels of asymmetric dimethylarginine. J Pharmacol Exp Ther. 2009;331:234–243. doi: 10.1124/jpet.109.153510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–H3245. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 37.Pharmaceuticals I. Clinical Trials using the FXR-agonist INT-747. 2011 www.interceptpharma.com.
- 38.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zorn JA, Wells JA. Turning enzymes ON with small molecules. Nat Chem Biol. 2010;6:179–188. doi: 10.1038/nchembio.318. [DOI] [PubMed] [Google Scholar]