Abstract
MyD88 is an adapter molecule that is used by both IL-1R and TLR family members to initiate downstream signaling and promote immune responses. Given that IL-1β is induced after S. aureus infections and TLR2 is activated by S. aureus lipopeptides, we hypothesized that IL-1β and TLR2 contribute to MyD88-dependent protective immune responses against post-arthroplasty S. aureus infections. To test this hypothesis, we used a mouse model of a post-arthroplasty S. aureus infection to compare the bacterial burden, biofilm formation and neutrophil recruitment in IL-1β-deficient, TLR2-deficient and wildtype mice. By using in vivo bioluminescence imaging, we found that the bacterial burden in IL-1β-deficient mice was 26-fold higher at 1 day after infection and remained 3- to 10-fold greater than wildtype mice through day 42. In contrast, the bacterial burden in TLR2-deficient mice did not differ from wildtype mice. In addition, implants harvested from IL-1β-deficient mice had more biofilm formation and 14-fold higher adherent bacteria compared with those from wildtype mice. Finally, IL-1β-deficient mice had ~50% decreased neutrophil recruitment to the infected postoperative joints than wildtype mice. Taken together, these findings suggest a mechanism by which IL-1β induces neutrophil recruitment to help control the bacterial burden and the ensuing biofilm formation in a post-surgical joint.
Keywords: Staphylococcus aureus, arthroplasty, joint, TLR2, IL-1β
INTRODUCTION
Despite the widespread use of intravenous antibiotic prophylaxis and a focus on aseptic surgical technique, post-arthroplasty infections still occur in ~1.2% of primary arthroplasties and 3–5% of revisions.1,2 The number of these infections is projected to increase to 266,000 per year by 2030 as the need for arthroplasty in the aging population will exceed 3.8 million surgeries.3,4 The treatment of a post-arthroplasty infection is extremely difficult, as invading bacteria form biofilms on implanted foreign materials that block penetration of immune cells and antibiotics.5,6 In the U.S., a two-stage surgical procedure is the standard treatment of care, which involves: (1) surgical removal of all prosthetic components with thorough debridement, placement of an antibiotic-impregnated spacer, administration of a 6-week course of intravenous antibiotics, and (2) revision arthroplasty after the infection has cleared.5,6 Taken together, the treatment of post-arthroplasty infection involves extensive medical and surgical care, enormous health care costs, prolonged disability/rehabilitation, and significantly worse outcomes.5,6
Staphylococcal species, including S. aureus and S. epidermidis, account for up to 70% of post-arthroplasty infections7,8 and an increasing proportion are due to methicillin-resistant S. aureus (MRSA).9 Recent evidence has demonstrated that humans and mice deficient in the signaling adapter molecule, myeloid differentiation factor 88 (MyD88), are highly susceptible to S. aureus infections.10,11 MyD88 signaling, which triggers a pathway that leads to NF-κB-mediated transcription of proinflammatory cytokines, chemokines and adhesion molecules, is activated by Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) family members.12,13 Relevant to S. aureus infections, TLR2 recognizes S. aureus lipopetides and lipoteichoic acid14,15 and IL-1β is induced during S. aureus infections,16,17 including S. aureus-infected joint tissue in patients.18 Furthermore, IL-1β plays a protective role in mouse and rabbit models of S. aureus septic arthritis.16,19 However, little is known as to whether these pathways that activate MyD88 are important for protective immunity against a post-arthroplasty S. aureus infection. Thus, we chose to evaluate the mechanism by which TLR2 and IL-1β play a role in host defense using an in vivo mouse model of post-arthroplasty S. aureus infection.
METHODS
Staphylococcus aureus bioluminescent strain
The bioluminescent S. aureus strain Xen36 (Caliper Life Sciences) was used in all experiments. This strain was derived from the parental strain ATCC 49525 (Wright), a clinical isolate obtained from a patient with S. aureus bacteremia. Xen36 emits a blue-green light with a peak at 490 nm because it contains the bioluminescent luxABCDE operon modified from Photorhabdus luminescens in a stable bacterial plasmid that is maintained in all progeny. This strain has been previously used to investigate S. aureus infections in models of bone allografts and osteomyelitis.20,21
Preparation of S. aureus for inoculation
Xen36 was streaked onto tryptic soy agar plates (tryptic soy broth [TSB] plus 1.5% bacto agar [BD Biosciences]) and grown at 37°C overnight as previously described.22 Single colonies of Xen36 were cultured in TSB and grown overnight at 37°C in a shaking incubator (240 rpm) (MaxQ 4450; Thermo). Mid-logarithmic phase bacteria were obtained after a 2 h subculture of a 1/50 dilution of the overnight culture. Bacterial cells were pelleted, resuspended and washed 3x in PBS. Bacterial concentrations were estimated by measuring the absorbance at 600 nm (Biomate 3; Thermo). Colony forming units (CFUs) were verified after overnight culture of plates.
Mice
12 week old male congenic mice on a C57BL/6 genetic background were used in all experiments. IL-1β–deficient mice (F8) have been previously described.17 TLR2-deficient mice (B6.129-Tlr2tm1Kir/J) (F7) and wildtype (wt) C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mouse colonies were maintained at UCLA in autoclaved cages under specific pathogen–free conditions.
Mouse surgical procedures
All procedures were approved by the UCLA Animal Research Committee. To model a post-arthroplasty S. aureus infection, an orthopaedic-grade stainless steel Kirscher-wire (K-wire) (0.6 mm in diameter; Synthes) was surgically placed into the right knee joint by accessing the distal right femur through a medial parapatellar arthrotomy as previously described.22 A femoral medullary canal was manually reamed with a 25-gauge needle and the K-wire was press-fit in a retrograde fashion and cut with 1 mm protruding into the joint space. An inoculum of Xen36 (1×103 CFUs) in 2 μl of saline was pipetted into the joint space containing the cut end of the implant and the surgical site was closed with Vicryl 5-0 sutures. Buprenorphine (0.1 mg/kg) was administered as an analgesic subcutaneously every 12 hours for 14 days. For in vivo bioluminescence, the mice were followed for 42 days. To evaluate biofilm formation, implanted pins were evaluated at an early (day 7) and late (day 42) time point. CFUs of bacteria adherent to the implant were determined on day 42. To evaluate neutrophil recruitment, which occurs early on after the inoculation, the joint tissue was harvested on day 1.
In vivo bacterial burden as measured by in vivo bioluminescence imaging
Mice (n=8 per group) were anesthetized with inhalation isoflurane (2%) and in vivo bioluminescence imaging was performed using the Xenogen IVIS Lumina® imaging system (Caliper Life Sciences) as previously described.22 Data are presented on color scale overlaid on a grayscale photograph of mice and quantified as maximum flux (photons per second (s) per cm2 per steradian (sr) [p/s/cm2/sr]) within a circular region of interest (1×103 pixels) using Living Image® software (Xenogen).
Variable-pressure scanning electron microscopy
Mice (n=3 per group) were euthanized on days 7 and 42, implants were harvested and biofilm formation on the intra-articular end of the implants were visualized using a field emission variable-pressure scanning electron microscope (VP-SEM) (FE-SEM Zeiss Supra VP40) as previously described.22
Quantification of adherent S. aureus bacteria on the implants
Bacteria adherent to the implants (n=5 per group) were quantified by detaching the bacteria from the implants harvested on day 42 by sonication in 1 ml 0.3% Tween-80 in TSB for 10 minutes followed by vortexing for 5 minutes and serial dilutions were plated and cultured overnight as previously described.22
Histologic analysis
Mice (n=3 per group) were euthanized on day 1 and infected joint tissue specimens were fixed in formalin (10%) overnight. Specimens were decalcified by incubation in Decalcifier II® solution (Surgipath) for 6 h and specimens were processed and embedded in paraffin. Sagittal sections (4 μm) were cut and stained with hematoxylin and eosin (H&E). Photomicrographs were obtained using a Leica DM2500 light microscope equipped with a DFC230 camera (Leica Microsystems).
Myeloperoxidase activity
Mice (n=5 per group) were euthanized on day 1 and infected joint tissue specimens were homogenized (Pro200® Series homogenizer; Pro Scientific). The tissue homogenate was centrifuged at 12,000 × g for 15 minutes at 4°C and supernatants were assayed for myeloperoxidase activity levels (ng/mg tissue) using the EnzChek® Myeloperoxidase Activity Assay Kit, according to the manufacturer’s instructions (Invitrogen).
Statistical analysis
Data were compared by using a Student’s t-test (two-tailed). All data are expressed as mean ± standard error of the mean (sem) where indicated. Values of p < 0.05 were considered statistically significant.
RESULTS
IL-1β-deficient mice had increased in vivo bacterial burden compared with TLR2-deficient mice or wt mice
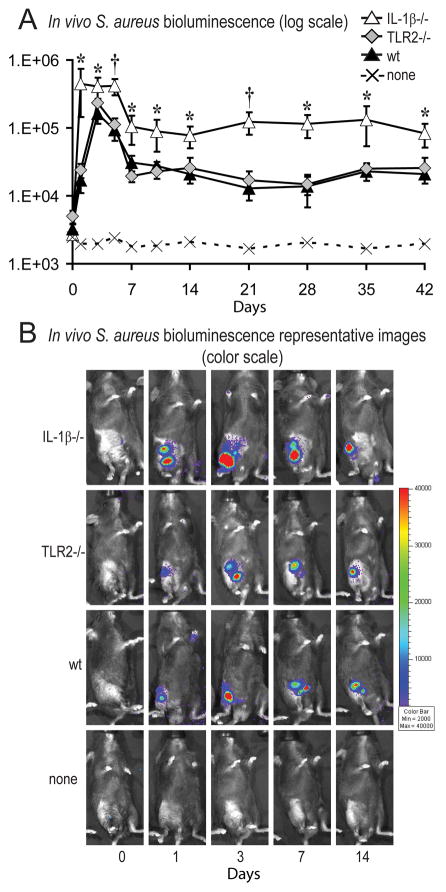
In the presence of an orthopaedic-grade implant, intraoperative knee joints of IL-1β-deficient, TLR-deficient and wt mice (n=8 per group) were inoculated with 1×103 CFUs of S. aureus strain Xen36, which contains a stable bioluminescent construct. In vivo bioluminescence imaging (Xenogen IVIS; Caliper Life Sciences) was used to determine the bacterial burden in vivo in anesthetized mice in real-time. Using this mouse model of post-arthroplasty infection, we previously determined that in vivo bioluminescence signals highly correlate with the bacterial CFUs harvested from infected knee joints.22 We found that IL-1β-deficient mice had a 26-fold greater bacterial burden compared with wt mice at day 1, which remained 3- to 10-fold greater than wt mice through day 42 (p<0.05) (Fig. 1). In contrast, the bacterial burden in TLR2-deficient mice did not differ from wt mice. These data demonstrate that IL-1β-deficient mice (but not TLR2-deficient mice) had higher bacterial burden than wt mice at all time points through postoperative day 42. Since the bacterial burden in TLR2-deficient mice did not differ from wt mice, the remaining experiments were designed to determine the mechanism for the higher bacterial burden observed in IL-1β-deficient mice.
Figure 1. IL-1β-deficient mice had increased in vivo bacterial burden compared with TLR2-deficient mice or wt mice.
The right knee joints of IL-1β−/−, TLR2−/− and wt mice were inoculated with 1×103 CFUs of S. aureus (n=8 mice/group) in the presence of an orthopaedic-grade K-wire implant. (A) Bacterial counts as measured by in vivo S. aureus bioluminescence (mean maximum flux [p/s/cm2/sr] ± sem) (logarithmic scale). †p<0.01, *p<0.05 IL-1β−/− versus wt mice. (B) Representative in vivo S. aureus bioluminescence on a color scale overlaid on top of a grayscale image of mice.
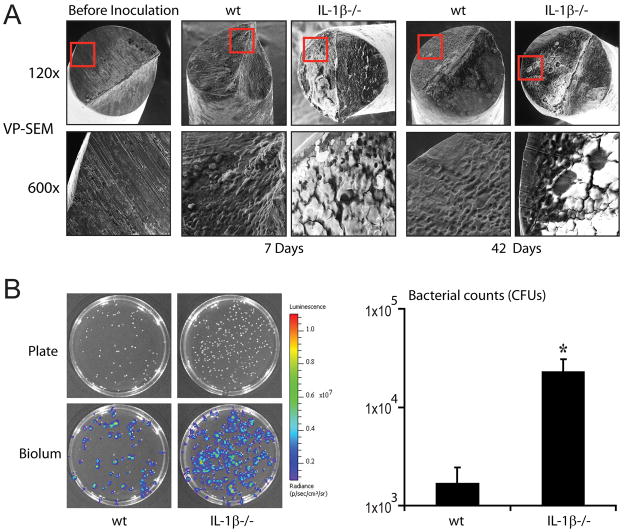
IL-1β-deficient mice had substantially more biofilm formation and adherent bacteria on the implants than wt mice
On postoperative days 7 and 42, implants harvested from IL-1β-deficient mice and wt mice (n=3 per group) had detectable biofilm formation as visualized by VP-SEM. However, IL-1β-deficient mice had markedly more biofilm formation than wt mice at both time points (Fig. 2A). To determine the numbers of bacteria present in the biofilms, implants (n=5 per group) were harvested at day 42 from IL-1β-deficient mice and wt mice (Fig. 2B). IL-1β-deficient mice had 14-fold higher bacterial CFUs adherent to the implants compared with wt mice (p<0.05). These results demonstrate that the more pronounced biofilms in IL-1β-deficient mice observed by VP-SEM harbor increased numbers of bacteria.
Figure 2. IL-1β-deficient mice had substantially more biofilm formation on the implants than wt mice.
The right knee joints of IL-1β−/− and wt mice were inoculated with 1×103 CFUs of S. aureus (n=3 mice/group) in the presence of an orthopaedic-grade K-wire implant. (A) Representative VP-SEM images of the biofilms on the intra-articular ends of the implants harvested from infected joints on postoperative days 7 and 42 are shown (1 of 3, with similar results). Top panels represent a low magnification (120x) and the bottom panels show a higher magnification (600x) of the area boxed in red. (B) Representative plates and bioluminescent colonies (left panels) and numbers of CFUs (right panel) of bacteria released from the implants (n=5 mice/group) on day 42 after overnight culture. *p<0.05 IL-1β−/− versus wt mice.
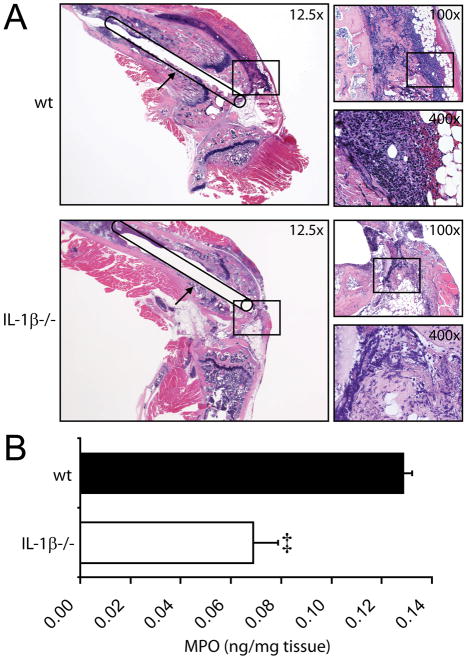
IL-1β-deficient mice had decreased neutrophil recruitment to the infected knee joints compared with wt mice
A critical first-line of defense is neutrophil recruitment to the site of a S. aureus infection, as demonstrated by the severe joint infections in mice depleted of neutrophils.19,23 To determine the degree of neutrophil recruitment, infected post-operative joint tissue from IL-1β-deficient and wt mice (n=3 per group) was obtained on day 1 and analyzed by histology (Fig. 3A). IL-1β-deficient mice had markedly less neutrophils within the infected joint tissue than wt mice. To quantify the number of neutrophils within the infected joints, myeloperoxidase (MPO) activity, which closely approximates neutrophil number, was determined on homogenized joint tissue from IL-1β-deficient and wt mice (n=5 per group) (Fig. 3B). IL-1β-deficient mice had ~50% less MPO activity in the infected joint tissue compared with wt mice (p<0.001). Thus, both histology and MPO assays demonstrated that IL-1β-deficient mice have significantly less neutrophil recruitment to the infected knee joints than wt mice.
Figure 3. IL-1β-deficient mice had decreased neutrophil recruitment to the infected knee joints compared with wt mice.
The right knee joints of IL-1β−/− and wt mice were inoculated with 1×103 CFUs of S. aureus in the presence of an orthopaedic-grade K-wire implant. The infected joint tissue was harvested on postoperative day 1. (A) Representative photomicrographs of histologic sagittal sections of wt mice (top panels) and IL-1β−/− mice (bottom panels) are shown (1 of 3 mice per group, with similar results). Left large panels: low magnification (12.5x) of H&E-stained joint specimens with a line drawing of the location of the implant extending into the joint from the femoral canal. Upper right small panels: higher magnification (100x) of H&E-stained joint specimens of the boxed area in the left panel at the location of the intra-articular end of the implant. Lower right small panels: higher magnification (400x) of H&E-stained sections in the boxed areas in the upper right panels. (B) Mean myeloperoxidase activity of the infected joint tissue specimens (ng/mg of tissue) ± sem (n=5 per group). ‡p<0.001 IL-1β−/− versus wt mice.
DISCUSSION
Infection after total joint arthroplasty is a disastrous complication. Treatment is extremely challenging and time-consuming, health care costs are enormous and patient outcomes are worse.5,6 While much time and energy has been spent on evaluating prophylactic strategies to prevent an infection,5,6 very little is known about the role of the immune response in combating these infections. Recent evidence has demonstrated that humans and mice deficient in MyD88 are highly susceptible to S. aureus infections.10,11 Therefore, we chose to evaluate the contribution of IL-1β and TLR2 in the immune response during a post-arthroplasty infection since they utilize MyD88 to initiate signaling and they have been previously implicated in host defense against S. aureus infections in various organs and tissues.16–19
Using a mouse model of post-arthroplasty S. aureus joint infection,22 we were able to study the roles of these proinflammatory mediators in both the early and late stages of the immune response to S. aureus in a post-surgical joint in the presence of a metallic implant. We found that IL-1β-deficient mice but not TLR2-deficient mice had markedly increased bacterial burden, which was most pronounced on day 1 but persisted through day 42. Furthermore, more prominent biofilm formation and higher numbers of adherent bacteria were observed on implants harvested from IL-1β-deficient mice compared with those from wt mice. Finally, analysis of MPO activity and histologic studies showed a significant decrease in neutrophil recruitment to the infected joints of IL-1β-deficient mice compared with those of wt mice.
Taken together, these findings demonstrate an important role for IL-1β in the early control of bacterial burden in a post-surgical joint. Furthermore, these data suggest a mechanism by which IL-1β mediates its protective effect through promoting neutrophil recruitment to the site of infection. This neutrophilic response likely contributes to controlling the bacterial growth and decreasing the ensuing biofilm formation. Interestingly, mice deficient in TLR2, which promotes immune responses through the same MyD88-signaling pathway as IL-1β, did not show an increased bacterial burden compared to wildtype mice. This finding is consistent with a recent report in humans demonstrating that a TLR2 polymorphism, which rendered this receptor dysfunctional, had no impact on the risk or outcome of post-arthroplasty S. aureus infections.24
It is tempting to speculate that manipulation of the IL-1β pathway could provide a therapeutic advantage to help prevent post-arthroplasty infections. However, enhancing the inflammatory response may have unwanted consequences in arthroplasty, especially since inflammatory cells (especially macrophage-induced inflammation) promote periprosthetic osteolysis that leads to implant loosening and failure.25 Indeed, IL-1β is upregulated in tissue surrounding failed total joint replacement implants and has been implicated in periprosthetic osteolysis.26–28 Thus, any therapeutic strategy would need to enhance the early protective IL-1β response while minimizing any sustained inflammation that would compromise the success of the implant.
Acknowledgments
We thank Ping Fu at the UCLA Tissue Pathology Core Laboratory (TPCL) for her expertise with embedding, cutting and H&E staining of joint biopsy sections. This work was funded by the Orthopaedic Hospital Research Center and by the UCLA Small Animal Imaging Resource Program (SAIRP) R24 CA92865 from the NIH.
References
- 1.Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 5.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 7.Fulkerson E, Valle CJ, Wise B, et al. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006;88:1231–1237. doi: 10.2106/JBJS.E.00004. [DOI] [PubMed] [Google Scholar]
- 8.Mittal Y, Fehring TK, Hanssen A, et al. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 9.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 10.Miller LS, O’Connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 11.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 16.Hultgren OH, Svensson L, Tarkowski A. Critical role of signaling through IL-1 receptor for development of arthritis and sepsis during Staphylococcus aureus infection. J Immunol. 2002;168:5207–5212. doi: 10.4049/jimmunol.168.10.5207. [DOI] [PubMed] [Google Scholar]
- 17.Miller LS, Pietras EM, Uricchio LH, et al. Inflammasome-Mediated Production of IL-1beta Is Required for Neutrophil Recruitment against Staphylococcus aureus In Vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 18.Varoga D, Klostermeier E, Paulsen F, et al. The antimicrobial peptide HBD-2 and the Toll-like receptors-2 and -4 are induced in synovial membranes in case of septic arthritis. Virchows Arch. 2009;454:685–694. doi: 10.1007/s00428-009-0780-4. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Matsukawa A, Ohkawara S, et al. Blocking of TNF-alpha and IL-1 inhibits leukocyte infiltration at early, but not at late stage of S. aureus-induced arthritis and the concomitant cartilage destruction in rabbits. Clin Immunol Immunopathol. 1997;82:18–25. doi: 10.1006/clin.1996.4276. [DOI] [PubMed] [Google Scholar]
- 20.Ketonis C, Barr S, Adams CS, et al. Vancomycin bonded to bone grafts prevents bacterial colonization. Antimicrob Agents Chemother. 2011;55:487–494. doi: 10.1128/AAC.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sottnik JL, U’Ren LW, Thamm DH, et al. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. 2010;59:367–378. doi: 10.1007/s00262-009-0755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernthal NM, Stavrakis AI, Billi F, et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS ONE. 2010;5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Helou O, Berbari EF, Brown RA, et al. Functional assessment of Toll-like receptor 2 and its relevance in patients with Staphylococcus aureus infection of joint prosthesis. Hum Immunol. 2010 doi: 10.1016/j.humimm.2010.10.001. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 25.Purdue PE, Koulouvaris P, Potter HG, et al. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 26.Epstein NJ, Warme BA, Spanogle J, et al. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. J Orthop Res. 2005;23:501–510. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 27.St Pierre CA, Chan M, Iwakura Y, et al. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 2010;28:1418–1424. doi: 10.1002/jor.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waddell J, Pritzker KP, Boynton EL. Increased cytokine secretion in patients with failed implants compared with patients with primary implants. Clin Orthop Relat Res. 2005:170–176. doi: 10.1097/01.blo.0000155079.29604.d4. [DOI] [PubMed] [Google Scholar]