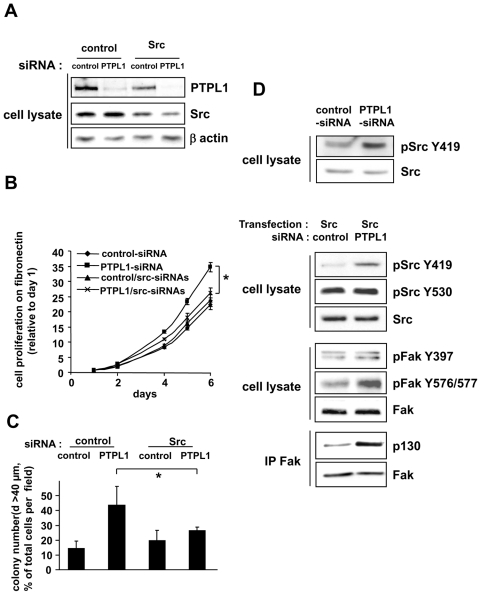
Figure 5. Implication of Src kinase activity for PTPL1 biological activity.
A, Five days after siRNA transfection, MCF-7 cells were lysed, and cell extracts were analyzed by immunoblot using anti-Src, anti-PTPL1 and anti-actin antibodies. B–C, One day after siRNA transfection, cells were plated on B) fibronectin and C) Matrigel. Outgrowth of transfected cells was monitored as described in Figure 2 C–D. The data given are the mean ± s.d. of B) triplicate C) quadruplet samples. One representative experiment out of three is shown. B) *P≤0.003. C)*P≤0.035. D, One day after siRNA transfection, MCF-7 cells were (second, third and fourth panel) or were not (first panel) transiently transfected with the Src expression vector. The phosphorylation state of specific tyrosine residues in Src (top blot) or FAK (middle blot) was monitored by western blot using anti-pSrcY419 and anti-pSrcY530 or anti-pFakY397 and anti-pFak576/577 antibodies. Equivalent amounts of Src or FAK expression were confirmed by re-probing the blots with anti-Src and anti-FAK antibodies. For Fak/P130 association (bottom blot), lysates were immunoprecipitated with anti-Fak antibody and immunoblotted with anti-P130cas. An equal immunoprecipitation level of FAK was confirmed by direct immunoblotting of the membrane after stripping with anti-Fak antibody.