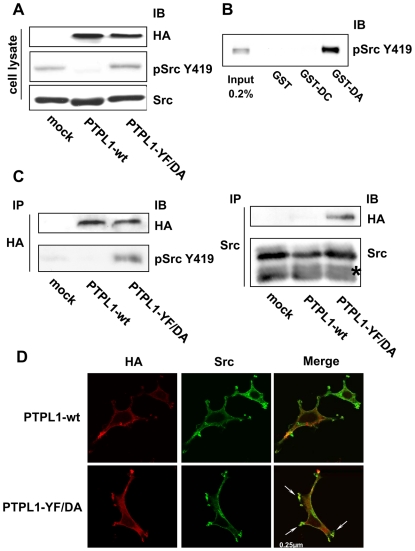
Figure 6. Phosphorylation and localization of Src kinase in PTPL1 transfected cells.
A, HEK293 cells were transiently cotransfected with Src and HA-tagged wt PTPL1 or PTPL1-YF/DA expression vectors. Total lysates were analyzed by direct immunoblotting with anti-pSrcY419 antibody (middle). Equivalent amounts of PTPL1 and Src were confirmed by re-probing the blots with anti-HA (top) and anti-Src (bottom) antibodies. B, HEK293 cells overexpressing the Src Y530F expression vector were serum-starved and stimulated with 10 mM pervanadate for 30 min. Cells were lysed and proteins were resolved by SDS-PAGE (7.5% gel; input) or incubated with the indicated GST fusion proteins immobilized on beads. Bound materials were immunoblotted with anti-pSrcY419 antibody. C, PTPL1 was immunoprecipitated from lysates prepared from HEK293 cells transfected as described in A) using an anti-HA antibody and immunoblotted for the presence of Src tyrosine-phosphorylated protein at residue 419 with anti-pSrcY419 antibody (bottom-left). Equivalent amounts of PTPL1 were confirmed by re-probing the blots with an anti-HA antibody (top-left). Src was immunoprecipitated from the same lysates using an anti-Src antibody and immunoblotted for the presence of PTPL1 using an anti-HA antibody (top-right). An equal expression level of Src was confirmed by direct immunoblotting of lysate with anti-Src antibody (bottom-right). Asterisks indicate IgG bands. D, Cells were treated for indirect FITC localization of PTPL1 constructs with anti-HA antibody (left) and indirect TRITC localization of Src (middle). Images represent merges of horizontal confocal sections (right). Bar, 250 nm.