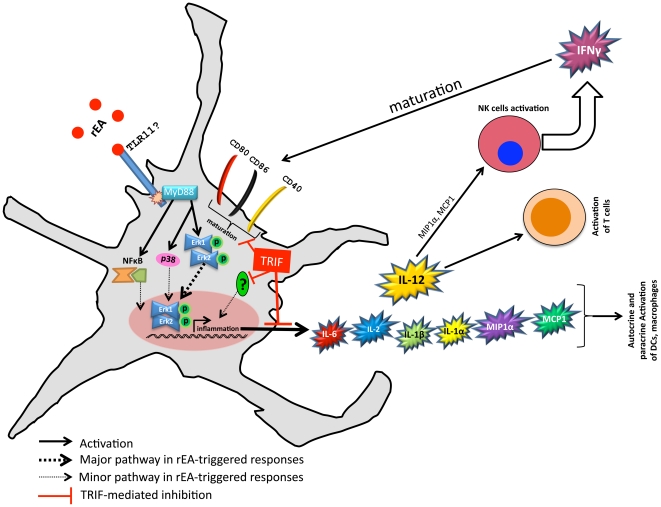
Figure 8. TRIF acts as a negative regulator of rEA-induced signaling and downstream responses in DCs: model of action.
rEA is a protein derived from Eimeria tenella [8], [11] and is highly homologous to Toxoplasma gondii profilin-like protein [14]. T. gondii has been shown to signal, at least in part, through TLR11; therefore, it is likely that TLR11 is one of the main pattern recognition receptors (PRRs) utilized by rEA. We have shown that rEA-triggered responses in vivo are completely dependent on MyD88. MAP kinases consequently become activated downstream of MyD88. Importantly, functional TRIF protein inhibited rEA-mediated signaling. DCs are a major cell type in mediating rEA responses and, under TRIF's inhibitory effects, have mitigated induction of surface expression of maturation markers and stunted release of pro-inflammatory cytokines/chemokines. Release of these molecules is critical for rapid amplification of immune responses and is mediated by autocrine and paracrine signaling [16]. We have confirmed that TRIF protein reduces release of pro-inflammatory cytokines/chemokines in response to rEA, resulting in reduced activation of NK, NKT, T, and B cells as well as reduced IFNγ production by NK cells.