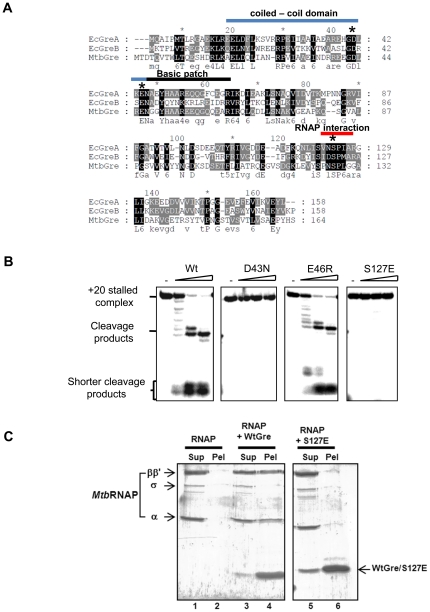
Figure 6. Conserved residues of MtbGre factor are important for Mg++ co-ordination and RNAP binding.
(A) Multiple sequence alignment of MtbGren (164 aa) with E. coli GreA (158 aa) and GreB (158 aa). N-terminal coiled-coil domain is marked in blue and its basic patch in black. The C-terminus RNAP interaction domain is marked in red in the alignment. Conserved acidic residues at the tip of N- terminus coiled coil domain and S127 at the C-terminus, subjected to site directed mutagenesis are marked by an ‘*’.(B) Comparative activity of Wt with D43N, E46R and S127E mutants in T7A1 TEC. (C) Ni-NTA pull down of his-tagged Wt and S127E mutant with MtbRNAP. Lane-1 and 2 represent the supernatant and pellet fraction from the control reaction having only MtbRNAP. Lanes 3,4 represent the supernatant and pellet fraction of WtGre respectively and lanes 5 and 6 represents mutant S127E along with MtbRNAP respectively.