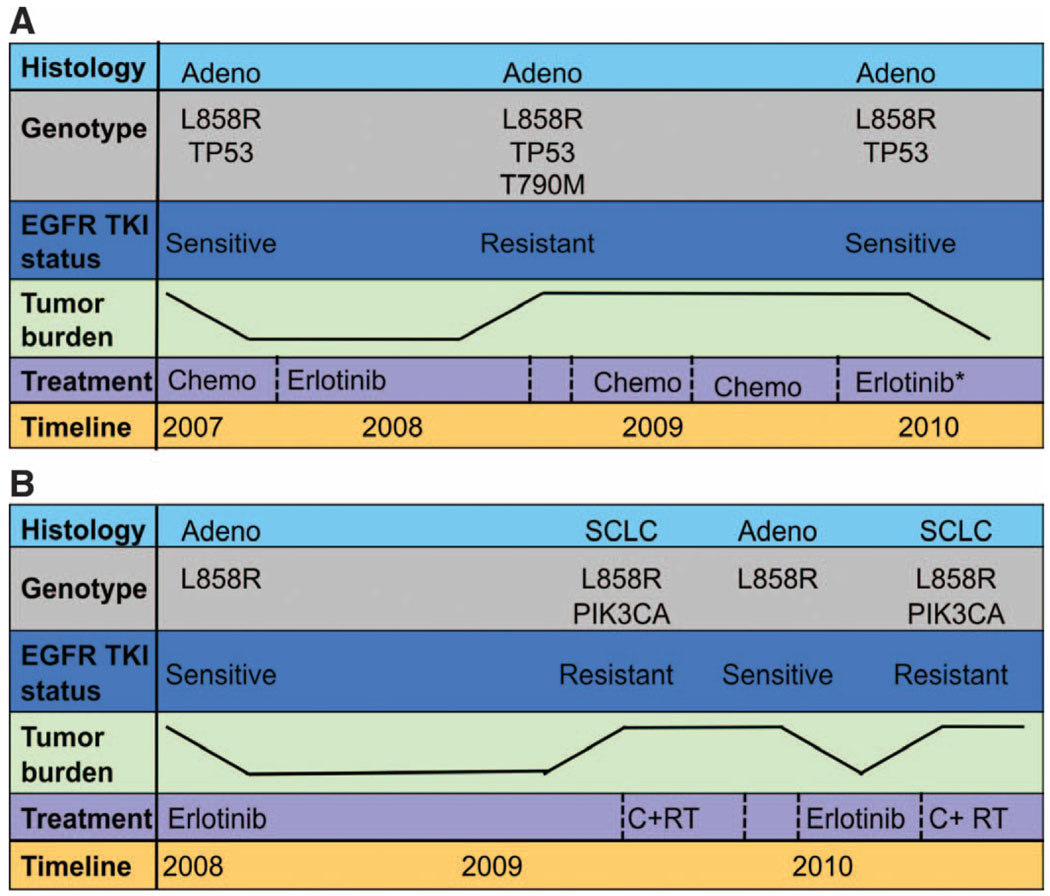
Fig. 5.
Longitudinal evaluation of patients treated repeatedly with erlotinib. The color-coded boxes to the left of each panel describe the data displayed across the timeline. The tumor burden depicted is not quantitative but represents tumor growth and shrinkage. (A) Patient 12 with a lung adenocar-cinoma carrying the L858R EGFR mutation and a mutation in the tumor suppressor p53 had a modest response to first-line chemotherapy. The patient then achieved a more robust and durable response to second-line erlotinib, with near-complete resolution of her lung nodules. After 8 months on an EGFR TKI, she developed resistance with growing bilateral pulmonary nodules. A lung core biopsy revealed an acquired T790M mutation in EGFR. There was no response to chemotherapy and she subsequently developed bone and liver metastases. At that time (after not taking the EGFR inhibitor for 8 months), a second lung core biopsy revealed the L858R EGFR mutation, but no detectable T790M EGFR resistace mutation. The patient responded to erlotinib (in combination with an investigational agent that did not target T790M). (B) Patient 24 had an L858R EGFR-mutant adenocarcinoma that responded markedly to first-line erlotinib for 12 months with resolution of her pleural effusion and pulmonary nodules. After 1 year, there was progression of the largest nodule. Core biopsy of this lesion revealed histological transformation to SCLC that harbored the EGFR L858R mutation and acquired a PIK3CA mutation. She was treated with radiation and chemotherapy. After a 6-month break from all treatment, her pleural effusion reaccumulated and small bilateral pulmonary nodules reappeared. Pleural effusion analysis revealed adenocarcinoma with the L858R EGFR mutation only (no PIK3CA mutation). Her disease responded to a second-line course of erlotinib. After 6 months, bony metastases and an adrenal lesion developed. Assessment of a growing bone metastasis revealed SCLC with both the L858R EGFR and PIK3CA mutations.