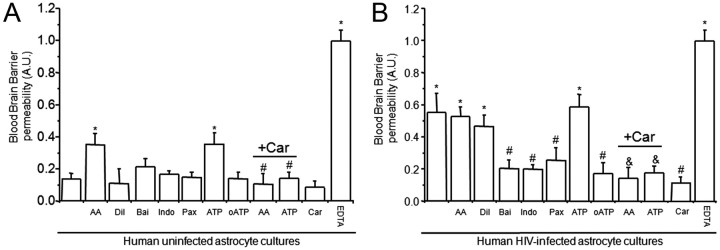
Figure 4.
Astrocyte end feet signaling participate in BBB disruption mediated by few HIV-infected astrocytes. We examined whether normal signaling pathways concentrated in astrocyte end feet that regulate blood flow are altered by HIVADA infection of astrocytes. We determined whether activation or blocking pathways used to control blood flow in the brain altered BBB integrity of our tissue culture model using albumin conjugated to Evans blue dye to assay for permeability. A, BBB cultures established with BMVECs and uninfected human astrocytes were totally impermeable. The addition of AA or ATP to the bottom of the BBB cultures established using uninfected astrocytes induced partial BBB disruption. Blocking activation of lipoxygenase and cyclooxygenase with baicalein (Bai) and indomethacin (Indo) on the astrocyte side did not alter BBB permeability when uninfected astrocytes were using. Blocking conductance calcium-activated potassium channels (BKCa) with paxilline (Pax) in the bottom of the BBB model, astrocyte side, did not alter permeability. Blocking ATP purinergic receptors using oxidized ATP (oATP) did not alter permeability of the barrier when uninfected astrocytes were used for the barrier. The disruption of the BBB induced by AA and ATP was GJ dependent because carbenoxolone (+Car) abolished disruption (n = 4; *p ≤ 0.005 compared with control and #p ≤ 0.003 compared with BBB inserts treated with AA or ATP). B, BBB cultures established using BMVECs and HIV-infected human astrocytes were highly permeable. The addition of AA, the diluent of AA (Tocrisolve), or ATP to the bottom of the BBB cultures did not change the already disrupted BBB. Blocking activation of lipoxygenase and cyclooxygenase using Bai and Indo on the astrocyte side was protective against BBB disruption induced by few infected astrocytes. Blocking BKCa with Pax in the bottom of the BBB model was also protective. Blocking ATP purinergic receptors using oATP (10 μm) was protective. This suggests the involvement of several signaling pathways in the BBB disruption induced by few HIV-infected astrocytes. The addition of carbenoxolone (+Car) to the bottom of the BBB cultures abolished BBB disruption induced by AA, ATP, or HIV-infected astrocytes (as shown in Fig. 2), indicating that gap junction channels play a key role in amplifying astrocyte end feet dysregulation resulting in BMVEC compromise and permeability (n = 4; *p < 0.005 compared with control uninfected conditions; #p < 0.003 compared with permeable BBB cultures established using HIV-infected astrocyte cultures; &p < 0.005 compared with treatment of BBB cultures containing HIV-infected astrocytes, treated with AA or ATP).