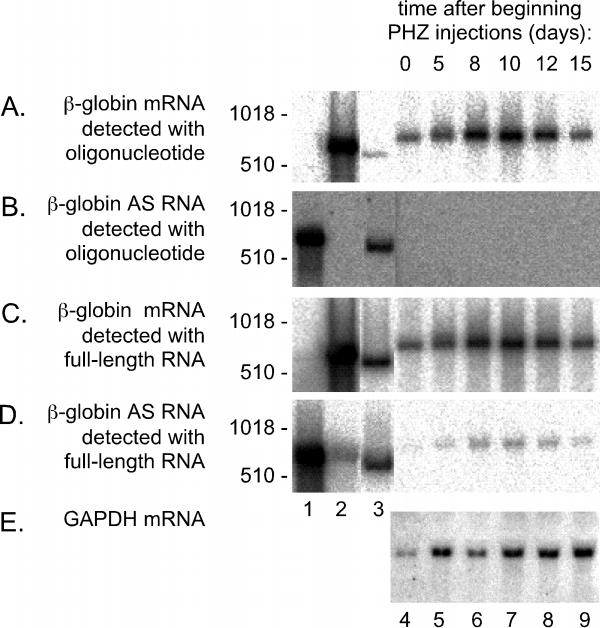
Figure 2.
Northern assays for sense and AS β-globin RNA in RNA extracted from spleens of anemic mice. Duplicate aliquots (30 μg) of RNA extracted from the spleens of animals as described for Fig. 1, were glyoxalated and electrophoresed into 2% agarose gels. After electrotransfer, the set of aliquots on one filter were hybridized with a 5'-labeled oligonucleotide to detect the sense strand of β-globin RNA (panel A). A second filter, with the same set of aliquots, was hybridized with a complementary oligonucleotide, to detect AS β-globin RNA (panel B). After quantitation of the radioactivity using a Bio-Imager, the two filters were stripped and rehybridized with radioactive full-length RNA probes to detect either the globin sense (panel C) or AS sequence (panel D). Again after quantitation of the radioactivity, the filter used in panels B and reused in D was stripped once more and rehybridized with an RNA probe to detect both the sense strands of GAPDH and 28 S rRNA (panel E). Lanes 1-3 represent hybridization standards of in vitro transcribed globin AS RNA (lane 1), globin sense RNA (lane 2), and double-stranded globin cDNA (lane 3). As expected, these three standards exhibit mobilities somewhat faster than that of mature globin mRNA. In all panels lanes 4-9 represent RNA from spleens of mice at 0, 5, 8, 10, 12, and 15 days, respectively, after the beginning of the PHZ injections. As size standards we used 5'-labelled DNA fragments, with sizes indicated at the left of each panel.