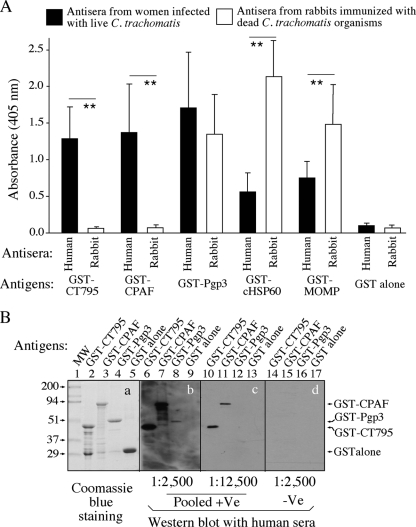
Fig. 1.
Recognition of the hypothetical protein CT795 by human and animal antibodies. (A) Reactivities were measured in an ELISA of various GST-fusion proteins, including CT795, CPAF (a Chlamydia-secreted serine protease), Pgp3 (a chlamydial outer membrane protein that is also secreted into host cell cytosol), chlamydial HSP60 (cHSP60), and MOMP, as well as GST alone, as indicated along the x axis, with antisera from 10 women who were urogenitally infected with live C. trachomatis organisms and 13 rabbits immunized with dead chlamydia organisms. The GST-fusion proteins were immobilized onto glutathione-coated microplates, and all antisera were used at a 1:500 dilution. The goat anti-human and rabbit IgG conjugates were used to detect primary binding, and the reactivities of the fusion proteins with the antisera were recorded as the absorbance at 405 nm (OD values displayed along the y axis). Note that GST-CT795 and GST-CPAF were preferentially recognized by human but not rabbit antisera, while the rest of the antigens were recognized by both human and rabbit antisera. (B) Pooled positive antisera (pooled +Ve; the 10 human antisera described for panel A pooled at an equal ratio) or pooled negative sera (pooled -Ve) from 8 women without C. trachomatis infection were used at various dilutions (as indicated at the bottom of the images) and reacted with GST-CT795, GST-CPAF, GST-Pgp3, or GST alone in a Western blot assay. Although the amounts of full-length fusion proteins loaded into the corresponding lanes were similar (lanes 2 to 5 in gel a), the human antibodies recognized both the denatured GST-CT795 (lanes 6 and 10) and GST-CPAF (lanes 7 and 11) better than the denatured GST-Pgp3 (lanes 8 and 12). Lane 1 was loaded with a prestained molecular weight marker (MW). **, P < 0.01 (ANOVA followed by Student's t test).