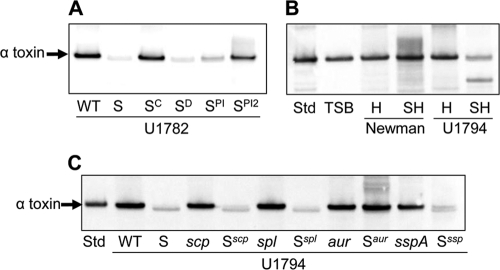
Fig. 5.
Impact of extracellular proteases on the alpha-toxin phenotype of sarA mutants. Western blots were done with anti-alpha-toxin antibody after blocking with both skim milk and human IgG. (A) Blots were done with stationary-phase supernatants from the USA300 isolate U1782 (WT) and its isogenic sarA mutant (S) complemented for the sarA defect (SC) or grown in the presence of DMSO (SD) or DMSO containing increasing concentrations of protease inhibitor cocktail (SPI versus SPI2). (B) Blots were done after adding purified alpha-toxin (8 μg per ml) to growing cultures of hla (H) and sarA hla (SH) mutants generated in strain Newman and the USA300 isolate U1794, the latter being derived from U1782 by curing the plasmid conferring resistance to erythromycin. This was necessary to allow generation of the sarA hla double mutant. After overnight incubation, supernatants were harvested for Western blotting using anti-alpha-toxin antibody. Controls included an equivalent amount of purified alpha-toxin standard (Std) incubated at 37°C overnight in sterile tryptic soy broth (TSB). (C) Blots were done with stationary-phase supernatants from U1794 (WT) and isogenic derivatives carrying mutations in sarA (S) with or without the indicated genes encoding extracellular proteases.