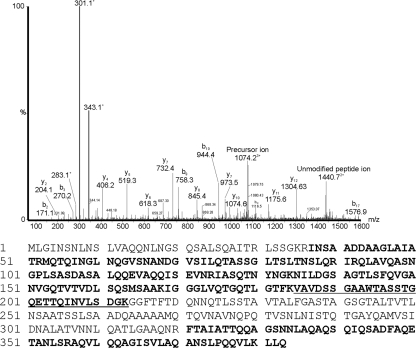
Fig. 4.
Mass spectrometry analyses of tryptic peptides from flagellin proteins of B. thailandensis E264. nLC-MS/MS spectrum of the triply protonated T185–213 glycopeptide ion of m/z 1074.2 from B. thailandensis. The spectrum is dominated in the low-molecular-m/z region by the carbohydrate oxonium ion (m/z 343.1) and what could be fragmentation components (m/z 301.1 and 283.1). From the observed mass of the glycopeptide, a mass excess of 342 Da was observed, corresponding to a single glycan modification. Below the graph is the amino acid sequence of the B. thailandensis E264 FliC protein. The sequence of the T185–211 glycopeptide is underlined. Sequences in bold indicate unmodified peptides observed in tryptic digests.