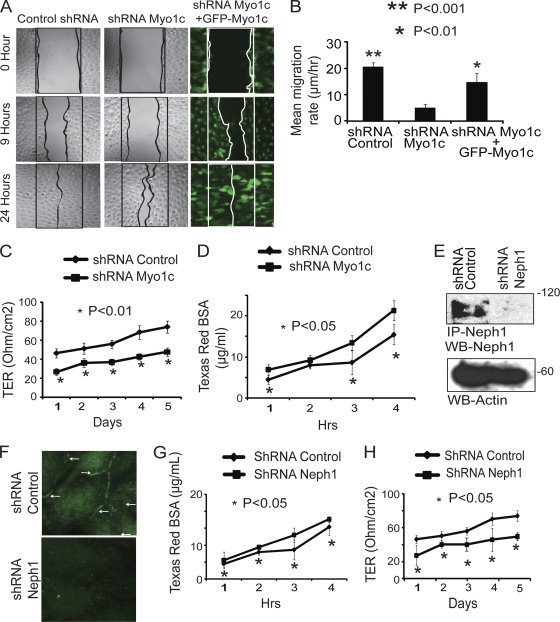
Fig. 11.
Depletion of Myo1c in podocytes results in decreased cell migration, reduced TER, and increased permeability for BSA. (A) A wound assay was performed with control cells, Myo1c knockdown podocytes, and Myo1c knockdown podocytes rescued with mouse GFP-Myo1c cDNA. Wound closure was observed at different time points (only data for 0 h, 9 h, and 24 h are presented). Unlike Myo1c knockdown cells, control and rescued cells showed complete wound closure in 24 h. (B) Quantitative analysis shows a significant reduction in the mean rate of migration of knockdown cells compared to control cells (P < 0.001) and rescued cells (P < 0.01). (C) Myo1c knockdown and control podocyte cells were grown on Transwell filters, and electrical resistance was measured. Myo1c knockdown cells showed reduced TER compared to that of controls (P < 0.05). (D) Control and Myo1c knockdown cells grown on Transwell filters were subjected to a paracellular albumin flux assay using Texas Red-labeled albumin. Increased albumin flux over time was observed for Myo1c knockdown podocytes compared to control cells (P < 0.05). (E and F) A plasmid encoding Neph1 shRNA was used to generate stable Neph1 knockdown cells, and the knockdown was assessed by immunoprecipitation and Western blotting (E) and immunostaining (F). (G and H) Similarly to Myo1c knockdown cells, albumin flux and TER analyses of Neph1 knockdown cells showed increases in albumin influx (G) and reductions in the TER (H) in Neph1 knockdown cells compared to controls (P < 0.05).