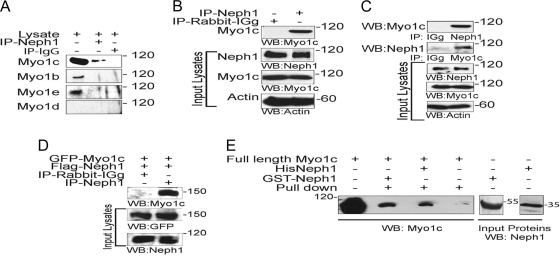
Fig. 2.
Neph1 interacts with Myo1c under in vivo and in vitro conditions. (A) Immunoprecipitation was performed with podocyte cell lysates using Neph1 antibody, and Western blotting was performed with antibodies specific to Myo1c, Myo1b, Myo1e, and Myo1d to determine their interactions with Neph1. (B) Neph1 was immunoprecipitated (IP) from rat glomerular lysates and immunoblotted with Myo1c antibody to confirm the interaction between Neph1 and Myo1c. (C) Reciprocal immunoprecipitation in cultured human podocytes using Myo1c antibody and Western blotting with Neph1 confirms the Neph1 and Myo1c interaction. (D) Plasmids encoding full-length GFP-Myo1c and Flag-Neph1 were transfected into COS-7 cells. Neph1 was immunoprecipitated from the cell lysate, and the immune complex was analyzed for Myo1c binding using Myo1c antibody. (E) The recombinant purified cytoplasmic domain of Neph1 as a GST or His fusion protein was mixed with purified full-length His-Myo1c expressed in baculovirus. Pulldown with either GST beads or Neph1 antibody and Western blotting with Myo1c antibody show binding between Neph1 and Myo1c.