Fig. 3.
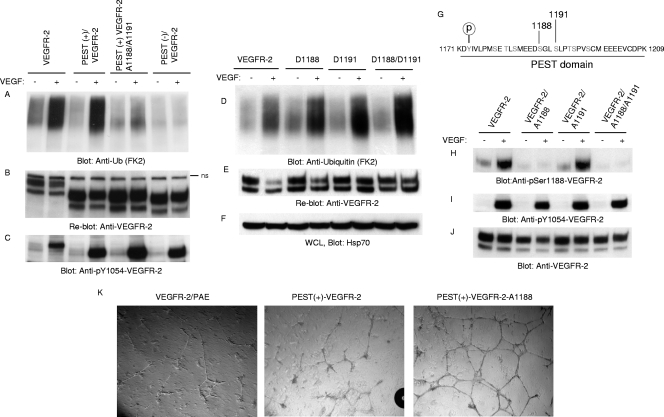
Phosphorylation of serine 1188 is required for ubiquitination of VEGFR-2. (A) VEGFR-2, PEST(+)VEGFR-2, PEST(−)VEGFR-2, and PEST(+)VEGFR-2/A1188/A1191 (where Ser1188 and Ser1191 were mutated to Ala) were stimulated with VEGF for 10 min (+) or were left unstimulated (−), and cells were lysed, immunoprecipitated with anti-VEGFR-2 antibody, and immunoblotted with antiubiquitin (Anti-Ub; FK2) antibody. (B) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (C) Whole-cell lysates were blotted for phospho-Y1054-VEGFR-2. (D) PAE cells expressing VEGFR-2, D1188/VEGFR-2, D1191/VEGFR-2, and D1188/D1191/VEGFR-2 (Ser-to-Asp mutation) were stimulated as described for panel A and blotted with antiubiquitin antibody. (E) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (F) Whole-cell lysates (WCL) shown in panel D were blotted with anti-Hsp70 for internal protein levels. (G) Putative phosphorylation sites in the PEST domain are shown. PAE cells expressing wild-type VEGFR-2, VEGFR-2/A1188, VEGFR-2/A1188/A1191, and VEGFR-2/A1191 were stimulated with VEGF, and whole-cell lysates were blotted with anti-phospho-Ser1188-VEGFR-2 antibody (H), phospho-Y1054-VEGFR-2 antibody (F), and anti-VEGFR-2 antibody (I). (J) The same membrane was reblotted with anti-VEGFR-2 antibody for protein levels. (K) PAE cells expressing wild-type VEGFR-2, PEST(+)VEGFR-2, or PEST(+)VEGFR-2/A1188 (in which serine 1188 is mutated to alanine) were plated on the 24-well plates coated with Matrigel in the presence of VEGF, and pictures were taken under a light microscope equipped with a digital camera.