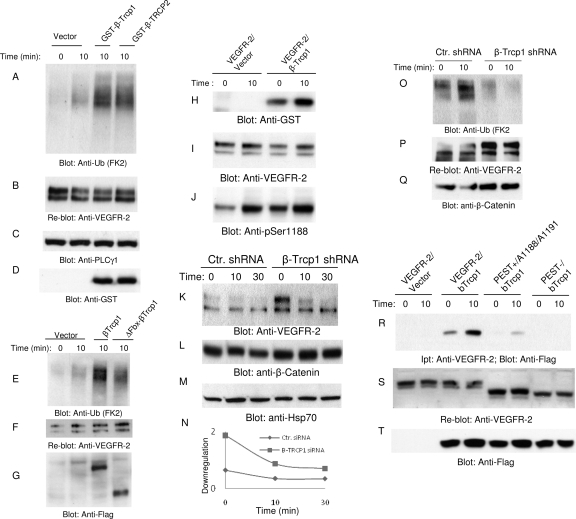
Fig. 4.
β-Trcp1 associates with VEGFR-2 and targets it for degradation. HEK293 cells expressing VEGFR-2 were transfected with an empty vector, GST-β-Trcp1, or with GST-β-Trcp2. (A) Cells were unstimulated (0) or stimulated with VEGF for 10 min, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with antiubiquitin (Anti-Ub; FK2) antibody. (B) The same membrane was reblotted with anti-VEGFR-2. Whole-cell lysates from panel A were blotted with anti-PLCγ1 (C) and anti-GST antibody (D). (E) HEK293 cells coexpressing VEGFR-2 with empty vector, with β-Trcp1, or with F-box-deleted β-Trcp1(ΔFbx-β-Trcp1) were left unstimulated (0) or stimulated for 10 min (8) with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with antiubiquitin antibody. (F) The same membrane was reblotted for VEGFR-2. (G) Whole-cell lysates (WCL) from the same lysates also were blotted for β-Trcp1 using anti-Flag antibody. (H) HEK293 cells coexpressing VEGFR-2 with empty vector or with β-Trcp1 were left unstimulated (0) or were stimulated for 10 min (10) with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with anti-GST antibody. (I) The same membrane was reblotted for VEGFR-2 levels. (J) Whole-cell lysates from the same cell lysate were blotted for phospho-Ser1188 antibody. Primary endothelial cells, HUVEC cells expressing pSuper.puro or shRNA.β-Trcp1.pSuper.puro, were stimulated with VEGF for the indicated periods of time, and whole-cell lysates were blotted with anti-VEGFR-2 antibody (K), β-catenin (L), and Hsp70 (M). Ctr., control. (N) Quantification of downregulation of VEGFR-2 is shown. (O) HUVEC expressing pSuper.puro vector or shRNA.β-Trcp1.pSuper.puro were left unstimulated (0) or were stimulated with VEGF for 10 min, and cells were lysed and immunoprecipitated with anti-VEGFR-2 and blotted with antiubiquitin antibody. The same membrane was reblotted for VEGFR-2 (P). Whole-cell lysates from the same cell lysates were blotted for β-catenin (L) and Hsp70 (Q). (S) HEK293 cells coexpressing VEGFR-2 with empty vector, VEGFR-2 with β-Trcp1, PEST(+)Ser1188/Ser1191 mutant VEGFR-2 with β-Trcp1, or PEST(−)VEGFR-2 with β-Trcp1 were unstimulated or stimulated with VEGF, lysed, immunoprecipitated with anti-VEGFR-2 antibody, and blotted with anti-Flag antibody. (T) The same membrane was reblotted for VEGFR-2. Ipt, immunoprecipitation. (U) Whole-cell lysates from the same cell lysates were blotted for β-Trcp1 using anti-Flag antibody.