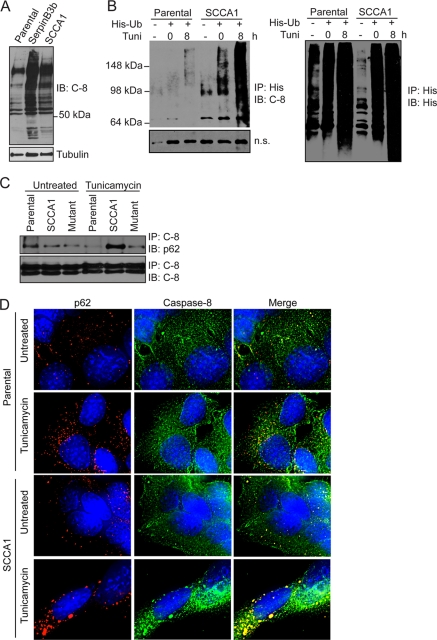
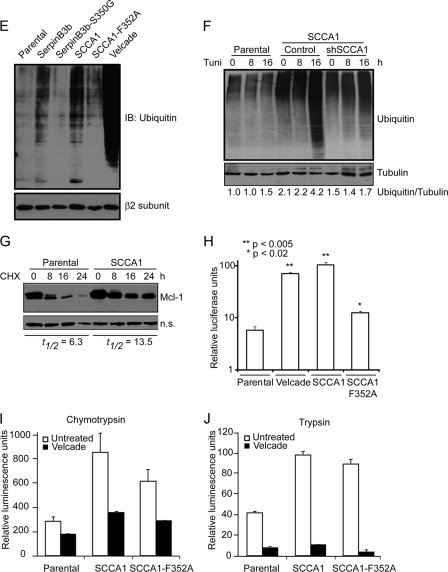
Fig. 6.
SCCA1 leads to increased caspase-8 ubiquitination and interaction with SQSTM1/p62. (A) bax−/− bak−/− BMK parental, SCCA1-expressing, and SerpinB3b-expressing cells were immunoblotted for caspase-8. Note the increase in higher-molecular-weight protein bands in SCCA1 or SerpinB3b cells, suggestive of polyubiquitination. (B) MCF10A parental and SCCA1-expressing cells were transfected with a polyhistidine-tagged Ub (His-Ub) and subsequently treated with tunicamycin for 8 h. Lysates were immunoprecipitated with a His antibody and blotted for caspase-8 or His tag. A nonspecific band (n.s.) was used to indicate equal loading. (C) Lysates from Hs578T parental or SCCA1-expressing cells, untreated or treated with tunicamycin for 24 h, were immunoprecipitated with a caspase-8 antibody and blotted for p62 or caspase-8. (D) SCCA1 promotes caspase-8 and p62 interaction upon ER stress treatment. bax−/− bak−/− BMK parental and SCCA1-expressing cells were left untreated or treated with tunicamycin (0.5 μg/ml) for 16 h. Immunofluorescence staining was performed using antibodies against p62 (red) or caspase-8 (green). (E) Cell lysates from untreated bax−/− bak−/− BMK parental or cells expressing wild-type or mutant serpins were examined for protein ubiquitination. Parental cells were treated with the proteasome inhibitor bortezomib (2 μM) for 8 h, as a positive control. The proteasome β2 subunit was probed for equal loading. (F) Parental and SCCA1 cells expressing an shControl or shSCCA1 were treated with tunicamycin. Cell lysates were subsequently examined for protein ubiquitination. The amount of ubiquitination was determined by densitometric analysis of the intensity of ubiquitination standardizing against tubulin. (G) Cells were treated for the indicated amount of time with cycloheximide (CHX; 10 μg/ml). Mcl-1, a protein degraded by the proteasome, was probed for, and the half-life (t1/2) was determined by densitometric analysis. (H) Parental, SCCA1, and SCCA1-F352A cells were transfected with a ubiquitin-luciferase reporter construct to determine the efficiency of degradation of ubiquitinated proteins. Parental cells were treated with bortezomib (2 μM) for 8 h as a positive control. Luciferase activity in cell lysates was determined, which allows for standardizing transfection efficiencies based on Renilla luciferase activity. (I and J) Parental cells and those expressing wild-type and mutant SCCA1 were left untreated or were treated with bortezomib (2 μM) for 8 h; proteasomal chymotrypsin-like (I) and trypsin-like (J) activities were assayed for using fluorogenic substrates.