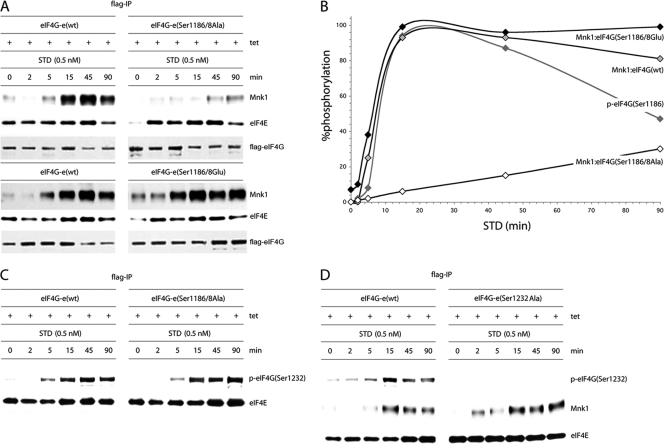
Fig. 7.
Kinetic analysis of STD-induced Mnk1 binding to wt eIF4G or its Ser1186/1188 double mutants. (A) Serum-starved, Tet-induced HEK293eIF4G-e cells or the corresponding eIF4G(Ser1186/1188Ala) and eIF4G(Ser1186/1188Glu) mutants were pretreated for 1 h with 10 μM CGP57380 and stimulated with 0.5 nM STD as indicated. Cell lysates were processed for co-IP of Mnk1 with Flag-IP of exogenous eIF4G. IP of Flag-eIF4G and co-IP of eIF4E are included as controls. This experiment was repeated three times; a representative assay is shown. (B) Chemiluminescence quantification of the kinetics of STD-induced Mnk1-eIF4G binding shown in panel A. The quantification of the kinetics of STD-stimulated eIF4G(Ser1186) phosphorylation reflects data shown in Fig. 5B. The interval with maximum signal was set at 100%, and the signal intensities at all other time points were calculated in relation to this. The data represent average percent phosphorylation values from three independent experiments. Standard deviation values were ≤4% at 0, 2, and 5 min, ≤6% or less at 15 and 90 min, and ≤7% at 45 min. (C) Phosphorylation of Ser1232 in eIF4G occurs upon STD stimulation of cells independent of Ser1186/1188 phosphorylation. IP of Flag-eIF4G-e in serum-starved, Tet-induced HEK293 cells expressing wt eIF4G-e or eIF4G-e(Ser1186/8A). (D) Time course of STD-stimulated Mnk1 co-IP in serum-starved, Tet-induced HEK293 cells expressing the eIF4G-e variants shown. The cells were pretreated for 1 h with 10 μM CGP57380 and stimulated with 0.5 nM STD as indicated. The experiment was repeated twice with similar results. A representative assay is shown.