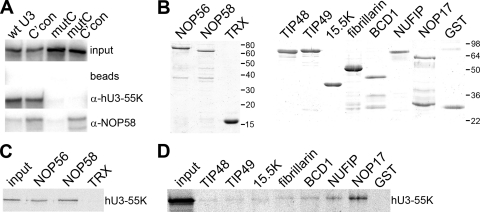
Fig. 3.
hU3-55K interacts with NOP56 and NOP58 in vitro. (A) U3 snoRNP complexes were assembled using wild-type and mutant 32P-radiolabeled RNAs in mouse nuclear extract. The mutations are indicated in Fig. 2B. RNP complexes formed during this reaction were immunoprecipitated using snoRNP-specific antibodies, and the copurifying RNAs were separated on an 8% polyacrylamide–7 M urea gel and then analyzed by autoradiography. The RNA used is indicated above each lane. Antibodies used for immunoprecipitation are indicated on the right. Input, 10% of the RNA after incubation in nuclear extract; beads, protein A Sepharose without antibodies. (B) Recombinant proteins used in protein-protein interaction studies were separated by SDS-PAGE and visualized by Coomassie staining. The protein loaded is indicated at the top of each lane. The positions of the marker proteins are indicated to the right of each panel. (C) Thioredoxin (TRX) and TRX fusion proteins of NOP56 and NOP58 were bound to anti-TRX antibody coupled to protein A Sepharose and then incubated with 35S-methionine-labeled, in vitro-translated hU3-55K. (D) Recombinant purified GST and GST-tagged proteins were bound to glutathione Sepharose and then incubated with 35S-methionine-labeled, in vitro-translated hU3-55K. Bound proteins were separated by SDS-PAGE and analyzed by autoradiography. The protein used is indicated at the top. Input: 10% of the 35S-labeled hU3-55K protein used in the interaction assay.