FIGURE 2.
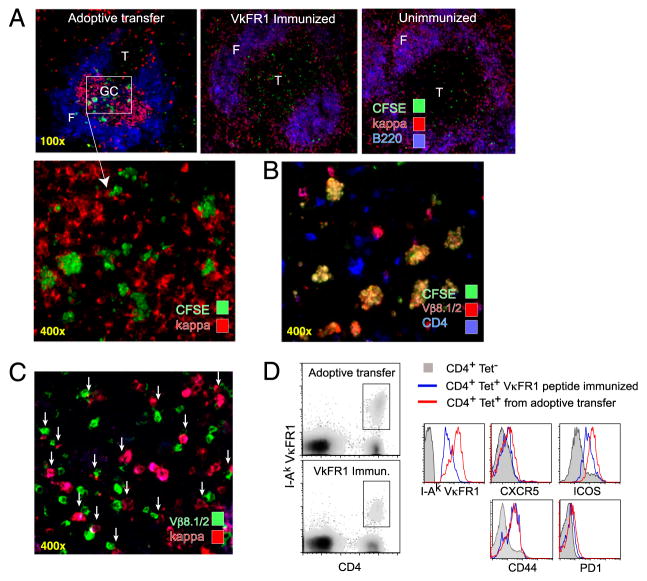
Rapid activation and migration of CA30 T cells to GC containing κTg B cells. A, Representative images of spleens from adoptive recipients of κTg cells (left), control VκFR1 peptide-immunized mice (center), or control unimmunized mice (right) that received enriched CFSE-labeled CA30 T cells (>90%) 50 h earlier. A/J recipient mice were used for VκFR1 peptide-immunization and no-immunization groups. Experimental group (top left) received CA30 T cells at day 7 following transfer of κTg splenocytes and immunization with GαMκ (alum). VκFR1 peptide immunization (alum) occurred at the time of CA30 transfer. CFSE staining of CA30 T cells was amplified with an Alexa-488–labeled anti-FITC mAb. B, CFSE+ cells in GC of mice that received κTg cells costain with both anti-CD4 and anti-Vβ8.1/2, as listed. C, Representative image of day 14 splenic GC from adoptive transfer recipients. Arrows highlight cognate interactions between κ+ B cells and Vβ8.1/8.2 T cells. D, Comparative expression levels of CXCR5, ICOS, CD44, and PD-1 among CD4+ I-Ak-VκFR1+ T cells 3 d after transfer of CA30 T cells. Experimental mice (red) received κTg B cells and were immunized with GαMκ (alum) 7 d before CA30 transfer. Control mice (blue) received CA30 T cells and were immunized with VκFR1 peptide in alum 3 d previously. Filled histogram represents CD4+ VκFR1 tetramer-negative T cells from experimental group. Results are representative of at least three independent experiments with three to five mice per group.