Figure 1. Biotransformation of tamoxifen and its metabolites.
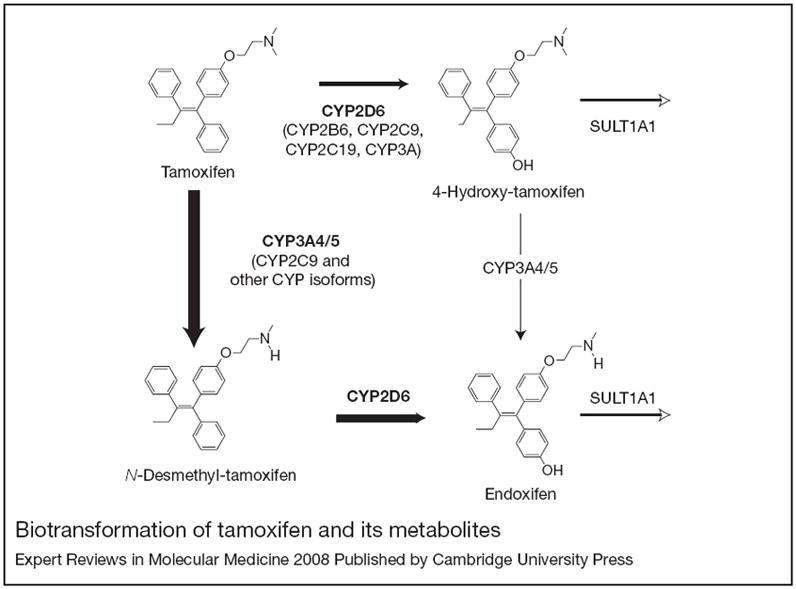
Cytochrome P450 (CYP) 3A4/5 is responsible for the formation of N-desmethyl-tamoxifen. The formation of 4-hydroxy-tamoxifen and of endoxifen are predominantly catalysed by CYP2D6. Other CYP isoforms, including CYP2C19, CYP2C9 and CYP2B6, appear to play less important roles in tamoxifen metabolism in vitro at therapeutically relevant concentrations. SULT1A1 is responsible for sulphation of 4-hydroxy-tamoxifen and may also play a role in endoxifen clearance. Reprinted, with permission from Oxford University Press, from Ref. 22 (© 2005 Oxford University Press).
