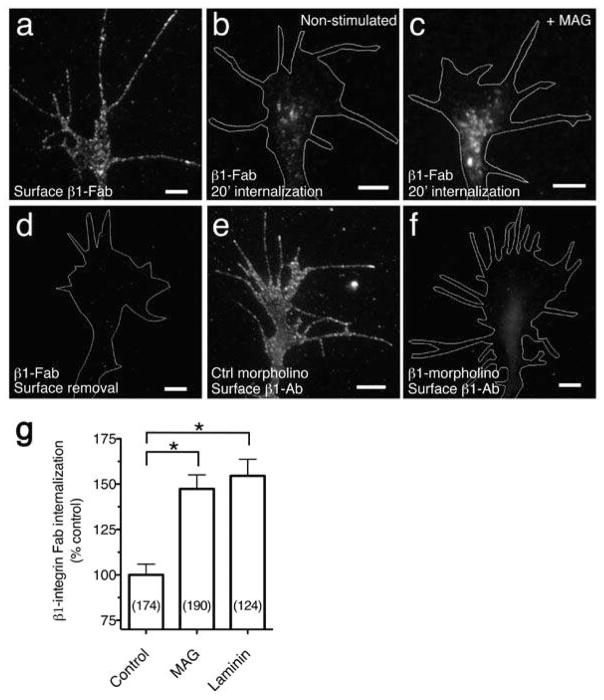
Figure 2. MAG stimulated endocytosis of β1-integrin.
(a) Fluorescence image shows β1 Fab specific to the extracellular domain of β1-integrin bound to the surface of a Xenopus spinal neuron growth cone at 8°C, prior to internalization or surface removal. (b,c) Endocytosed β1 Fab after surface binding as in (a) and treatment with BSA vehicle (b) or MAG (1 μg/ml; c) during a 20-min internalization period. Any remaining surface-bound Fab was removed with a low pH wash. (d) Removal of surface-bound β1 Fab with a low pH wash at 8°C, prior to internalization. (e,f) β1-antibody specificity assessed by immunofluorescence labeling of neurons cultured from embryos that were injected with control (e) or β1-integrin (f) morpholinos to knock down expression (quantitation in Supplementary Fig. 3. Scale bars, 5 μm. (g) Summary of β1 Fab internalization measured by the mean fluorescence intensity of growth cones treated with BSA vehicle, MAG, or soluble laminin (25 μg/ml) during the 20-min internalization period. Data are the mean ± s.e.m. from 3 independent experiments (n = the number associated with each bar; * P < 0.0001, bracketed comparisons, Mann-Whitney U-test).